Nickel is usually found in low-valent states. Higher-valent nickel species (i.e., tri- or tetravalent) are proposed to act as intermediates in nickel-catalyzed reactions and exist in metalloenzymes. The tetravalent state had been the highest known valence for this element so far, with only a few known examples of NiX4-type complexes.
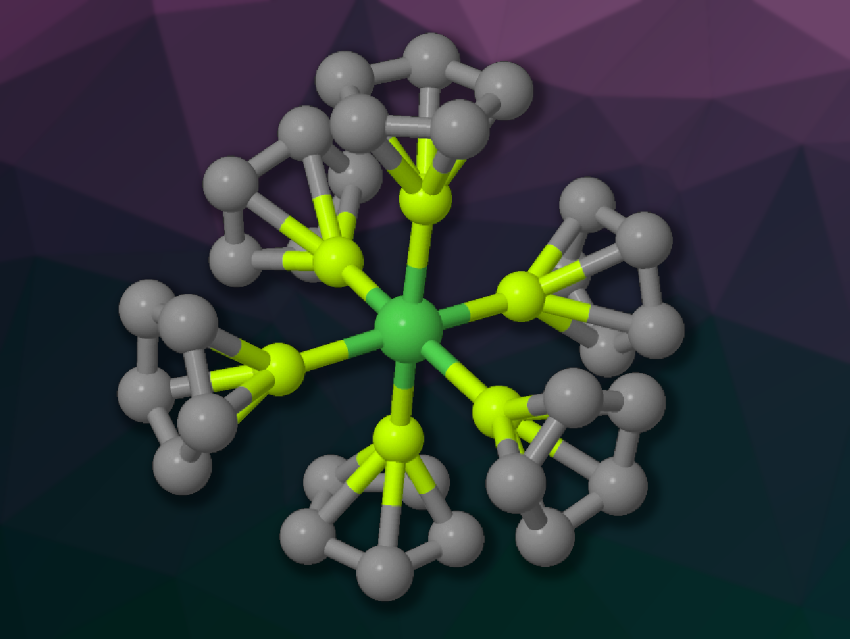
Josef T. Boronski, Imperial College London, UK, Simon Aldridge, University of Oxford, UK, and colleagues have synthesized a stable, crystalline hexavalent nickel (NiX6-type) complex, Ni(BeCp)6 (pictured, Cp cyclopentadienyl), using “beryllyl” ([BeR]−) ligands. The team reacted diberyllocene (BeCp)2 with bis(cyclooctadiene)nickel(0) (Ni(COD)2 in benzene. The product was obtained in the form of colorless, needle-shaped crystals in a yield of 91 %.
Single-crystal X-ray crystallography confirmed that the reaction gave the hexavalent complex Ni(BeCp)6. The team attributes the formation of this unique complex to the electronic and steric properties of the beryllyl ligands, which have a strong σ-donating character. Quantum-chemical calculations showed that the complex features seven-center, two-electron delocalized bonding across the NiBe6 core, with nucleus-independent chemical shift (NICS) calculations indicating a possible stabilizing aromatic character.
- A Crystalline NiX6 Complex,
Josef T. Boronski, Agamemnon E. Crumpton, Simon Aldridge,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c12125