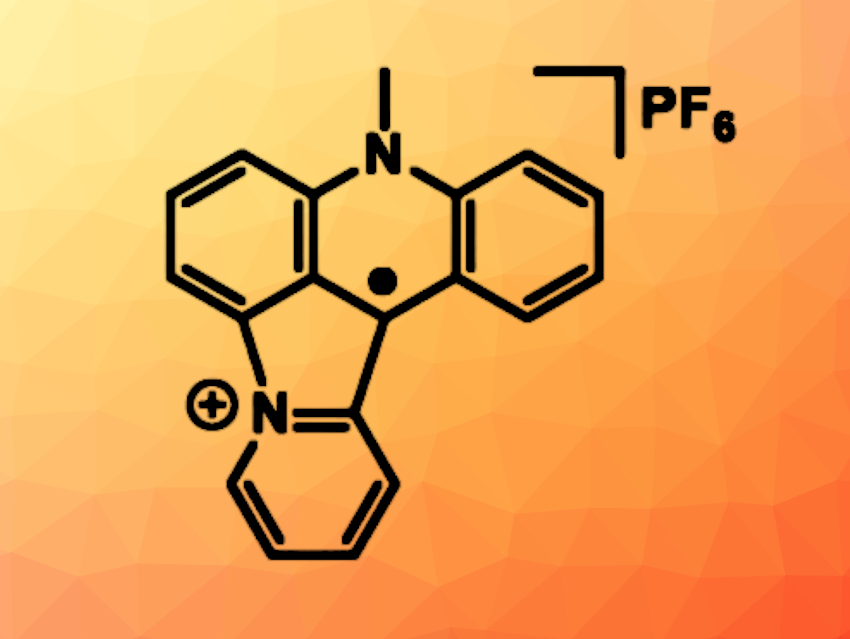
Stable organic radicals are challenging to synthesize. Known examples are often stabilized kinetically by the introduction of bulky substituents. Thermodynamically stable radicals without this steric stabilization could be useful in functional materials. Acridinium derivatives, i.e., pyridinium species extended by two benzene rings fused to either side, are interesting, e.g., for their photophysical and photochemical properties.
Henri-Pierre Jacquot de Rouville, Christophe Gourlaouen, Université de Strasbourg, France, and colleagues have synthesized a stable acridinium-based radical (hexafluorophosphate salt pictured), which they call “viridium”, referring to the radical’s greenish color and based on the Latin for green (viridis). The team obtained this stable organic radical via an intramolecular photocyclodehydrogenation of the corresponding 10-methyl-9-(pyridin-2-yl)acridinium cation under a Xe lamp.
The obtained radical is stable under air at room temperature. It can undergo reversible redox reactions, i.e., further oxidation to the corresponding dication or reduction to a neutral species. In solvents such as water, the radical undergoes a spontaneous π-dimerization. The researchers propose that this type of stable radical could have applications, e.g., in molecular machines or bioimaging.
- Viridium: A Stable Radical and Its π-Dimerization,
Henri-Pierre Jacquot de Rouville, Christophe Gourlaouen, David Bardelang, Nolwenn Le Breton, Jas S. Ward, Laurent Ruhlmann, Jean-Marc Vincent, Damien Jardel, Kari Rissanen, Jean-Louis Clément, Sylvie Choua, Valérie Heitz,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c13807



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)