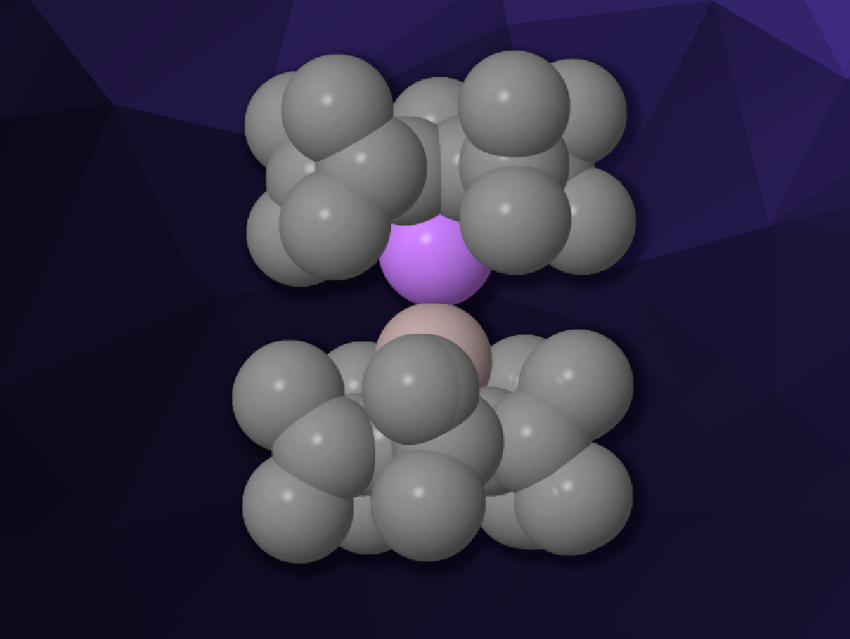
Ferrocene (Fe(C5H5)2) is a well-known example of a sandwich complex, consisting of two cyclopentadienyl ligands bound to a central iron atom. There is a range of other metallocene complexes with a variety of different metals in place of the iron center. Dimetallocenes are rare complexes with two metal atoms “sandwiched” between cyclopentadienyl-type ligands. Known examples of this type of compound are decamethyldizincocene (Cp*2Zn2, Cp* = 1,2,3,4,5-pentamethylcyclopentadienyl) and diberyllocene (Cp2Be2). Dimetallocenes of p-block elements and heterobimetallic dimetallocenes, in which two different metals are sandwiched, have remained experimentally unknown so far.
André Schäfer, Saarland University, Saarbrücken, Germany, and colleagues have synthesized such an elusive heterobimetallic dimetallocene, i.e., a lithium–aluminium dimetallocene with sterically demanding pentaisopropylcyclopentadienyl (5Cp) ligands (pictured). The team first prepared the monomeric cyclopentadienylaluminylene (5Cp)Al: by reacting the tetramer (Cp*Al)4 with 5CpLi∙OEt2. The resulting aluminylene was reacted with one equivalent of 5CpLi∙OEt2 in the presence of Cp*Li to capture Et2O, and the team obtained the desired lithium–aluminium dimetallocene 5CpLi–Al5Cp.
The new complex was characterized using single-crystal X-ray diffraction. The team obtained crystal structures of the complex co-crystalized with either toluene or 1,2-difluorobenzene. The ligands are bonded in an η5 manner in a staggered configuration. The distance between the Al and Li atoms is significantly shorter than in ionic aluminyl lithium complexes, and it is in good agreement with the predicted sum of the covalent radii of Al and Li.
Density functional theory (DFT) calculations indicate that that complex features a polar dative bond, with the lone pair at the aluminium atom as the donor and an empty orbital at the lithium atom as the acceptor. The Al–Li bond is relatively weak, and the complex is stabilized by dispersion interactions between the ligands. When the new complex is reacted, for example, with an N-heterocyclic carbene (NHC) ligand, the Al–Li bond is broken and a 5CpLi∙NHC complex is formed.
- A lithium–aluminium heterobimetallic dimetallocene,
Inga-Alexandra Bischoff, Sergi Danés, Philipp Thoni, Bernd Morgenstern, Diego M. Andrada, Carsten Müller, Jessica Lambert, Elias C. J. Gießelmann, Michael Zimmer, André Schäfer,
Nat. Chem. 2024.
https://doi.org/10.1038/s41557-024-01531-y