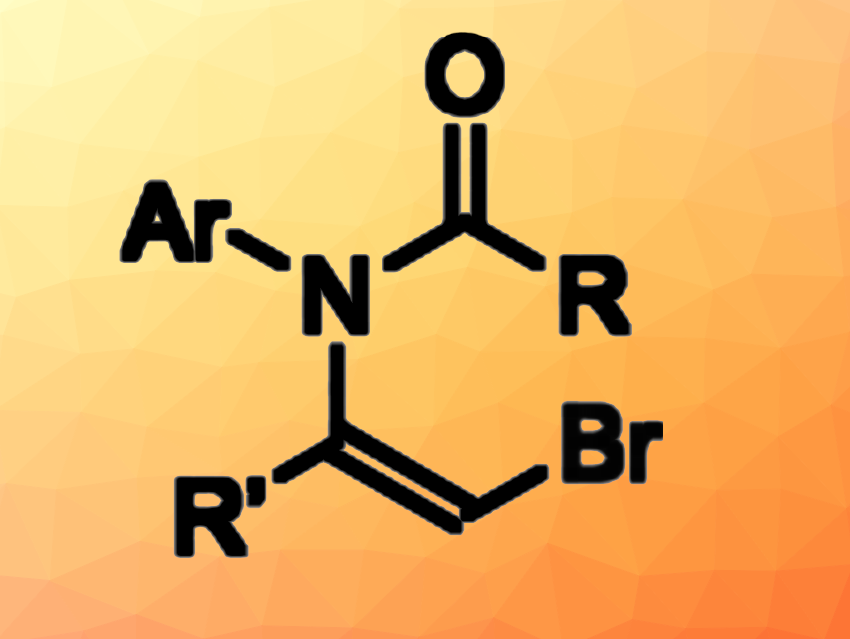
β-Halovinyl amides are useful intermediates in organic synthesis with multiple functional groups that can be used for further functionalizations. Existing methods for their synthesis can have drawbacks such as limited regioselectivity or a requirement for electron-deficient alkene units.
Masayuki Shigeta, Hirokazu Urabe, Tokyo Institute of Technology, Yokohama, Japan, and colleagues have developed a solvent- and transition-metal-free method for the regioselective synthesis of (Z)-β-halovinyl amides (general product structure pictured). The team’s approach is based on the nucleophilic addition reaction of amides to haloalkynes. They reacted these building blocks in the presence of K3PO4 without a solvent at 120 °C.
Under these conditions, the researchers obtained the desired (Z)-β-halovinyl amides in moderate to high yields. The team proposes a reaction mechanism that involves the deprotonation of the amide by K3PO4, followed by a nucleophilic attack on the haloalkyne to give a vinyl anion. This anion is then protonated by K2HPO4 or an amide molecule. The products can serve as precursors, e.g., for alkylidene carbenes and allyl halides, which are useful in further transformations.
- Nucleophilic Addition of Amides to Haloalkynes: Synthesis of (Z)-β-Halovinyl Amides as Dual Precursors of Alkylidene Carbenes and Allyl Halides,
Azusa Ishii, Takeshi Hata, Masayuki Shigeta, Hirokazu Urabe,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c00589