The reduction of nitric oxide (NO) is an important reaction in biology. For example, bacterial NO reductases (NORs) can reduce NO to N2O. Flavodiiron NO reductases (FNORs) in pathogens can use this reaction to provide resistance to the human immune defense agent NO. These enzymes have non-heme diiron active sites. Identifying intermediates in the reduction of NO at these sites could provide useful insights. The results of computations indicate that iron-hyponitrite complexes could be key species in this process. However, there is little information about the coordination chemistry of non-heme iron centers with hyponitrite (N2O22−).
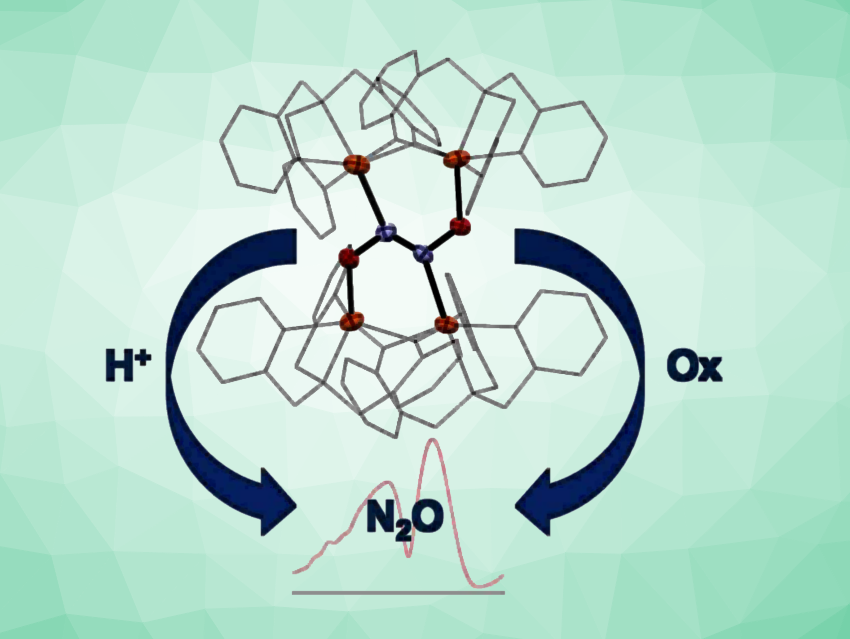
Nicolai Lehnert, The University of Michigan, Ann Arbor, USA, and colleagues have synthesized a non-heme iron hyponitrite complex (simplified structure pictured). The team first reacted the complex [FeII(TPA)(MeCN)2](OTf)2 (TPA = tris(methylpyridyl)amine, OTf– = triflate) with a sodium trans-hyponitrite hydrate (Na2N2O2 • x H2O) in methanol. They found that this led to the cleavage of the hyponitrite and the formation of the dimeric complex [Fe2(TPA)2(OMe)2](OTf)2. When they reacted a complex with a less Lewis-acidic iron center, i.e., [Fe2(BMPA-PhO)2(OTf)2] (BMPA-PhOH = (2-((bis(pyridin-2-ylmethyl)amino)methyl)phenol), with Na2N2O2 in acetonitrile as a polar aprotic solvent, they found that the tetranuclear iron-hyponitrite complex [{Fe2(BMPA-PhO)2}2(μ-N2O2)](OTf)2 was formed.
The product structure was confirmed using X-ray crystallography. The hyponitrite ion is bound to the four iron centers in the tetramer by both of its N and O atoms. According to the researchers, this represents a unique binding mode that had not been observed before. They also observed that upon the addition of acid to the tetrameric hyponitrite complex, quantitative N2O formation took place. This indicates that protonation could be key for hyponitrite activation and N2O formation. Oxidation of the iron centers also induced N2O formation by modulating the Lewis acidity.
- Synthesis and Structural Characterization of a Non‐Heme Iron Hyponitrite Complex,
Michael Lengel, Hai Dong, Nicolai Lehnert,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202409700