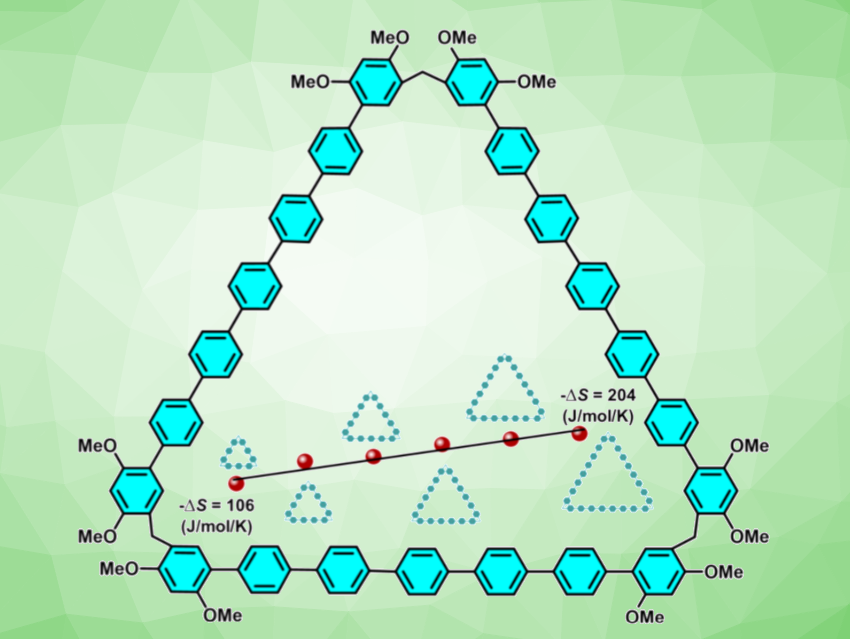
Macrocycles are useful, e.g., for molecular recognition or applications involving self-assembly. However, many available macrocyclic compounds have cavities with diameters smaller than 1 nm, limiting their range of applications in supramolecular chemistry. The efficient synthesis of giant macrocycles remains a significant challenge, because an increase in the monomer number results in entropy loss upon cyclization.
Zhi-Yuan Zhang, Chunju Li, Tianjin Normal University, China, and colleagues have developed a low-entropy-penalty synthesis strategy for producing giant macrocycles (p-oligophen[n]arenes, example pictured) in high yields. In this process, long and rigid monomers with two 2,4-dimethoxyphenylboronic acid reaction sites were condensed with paraformaldehyde via Friedel–Crafts reactions. Using this approach, a series of giant macrocycles with cavity sizes ranging from 2.0 to 4.7 nm were synthesized, with cyclization yields of up to 72 %.
Extending the monomer length, rather than increasing the monomer number, reduces the entropy penalty upon cyclization and avoids configuration twists, thereby favoring the formation of giant macrocycles with large cavities. These giant macrocycles can assemble into organogels in various solvents and show photoluminescence quantum efficiencies of up to 83.1 %.
According to the team, the work provides an effective and general method for preparing giant macrocycles. Thus, it expands the supramolecular toolbox for host–guest chemistry and assembly applications.
- Low‐entropy‐penalty synthesis of giant macrocycles for good self‐assembly and emission enhancement,
Xiao‐Na Sun, Ao Liu, Kaidi Xu, Zhe Zheng, Kai Xu, Ming Dong, Bo Ding, Jian Li, Zhi‐Yuan Zhang, Chunju Li,
Aggregate 2024.
https://doi.org/10.1002/agt2.607