Rebecca Freeman and Michael Bollong, The Scripps Research Institute, CA, USA, have found a surprising effect of the drug HPPE (N-(2-(2-hydroxyethoxy)ethyl)-1-methyl-
2-((6-(trifluoromethyl)benzo[d]thiazol-2-yl)amino)-1H-benzo[d]imidazole-5-carboxamide).
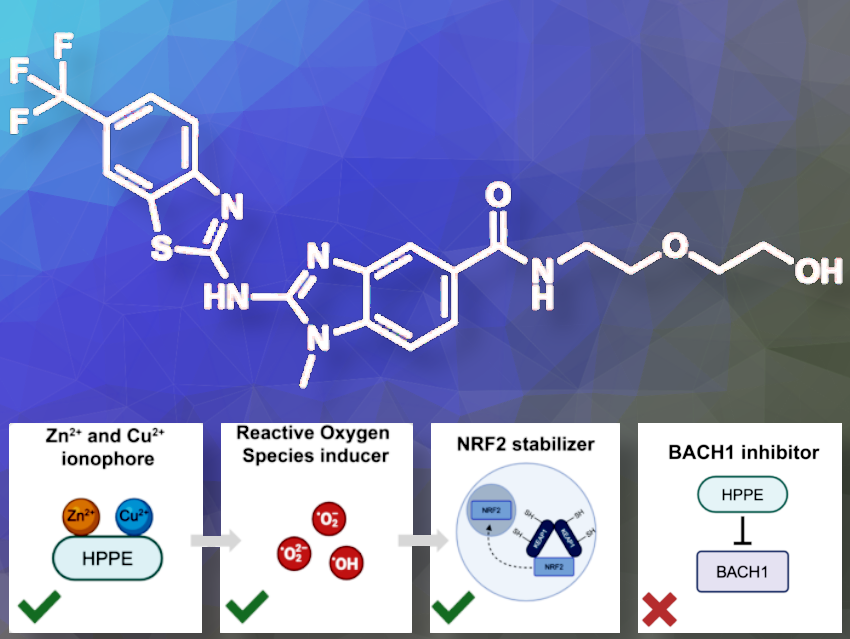
BACH1 is a protein that blocks the production of genes that help protect cells from oxidative stress and thereby the damage caused by harmful molecules. BACH1 overexpression has been associated with several disease states, including Alzheimer’s Disease, Down Syndrome, and pancreatic cancer. Scientists thought HPPE inhibits BACH1, which would alleviate cell stress. Instead, HPPE activates another protein called NRF2, which helps protect cells. HPPE works by releasing zinc inside the cells and causing a small, non-harmful amount of stress, which turns off another protein called KEAP1 that usually blocks NRF2.
Can you summarize in your own words what you did?
In this paper, we uncover an alternative mechanism for how the reported BACH1 inhibitor induces an antioxidant transcriptional program in cells. To our surprise, we found that HPPE does not depend on BACH1, but instead acts as an ionophore. It liberates Zn2+ and/or Cu2+ stores in cells and induces low levels of oxidative stress, which in turn activates NRF2.
What interested you about this?
We were interested in better understanding the mechanism through which HPPE operates. The paper that originally reported HPPE as a BACH1 inhibitor offered limited insight into the mechanism, thus creating an opportunity for our group to explore this in greater detail.
What is new and cool about your work?
HPPE is a small molecule that activates the oxidative stress response in a previously uncharacterized manner. Typically, NRF2 activators are electrophilic chemicals that covalently inhibit the target KEAP1, a negative regulator of NRF2. Instead of covalently modifying one of the ‘sensor’ cysteines of KEAP1, HPPE acts as an ionophore, ferrying levels of heavy metals from other cellular compartments into the cytoplasm. Increased heavy metal levels result in a nonlethal amount of oxidative stress, which is sensed by KEAP1 via oxidation of its sensor cysteine residues, resulting in stabilization of NRF2 and activation of its transcriptional program.
What is the main significance of your results?
Our findings principally suggest that HPPE, the basis for the small molecule clinical candidate ASP8731, is not a BACH1 inhibitor, as previously reported. It acts through a distinct mechanism.
Additionally, the work demonstrates that hormesis-inducing agents, like HPPE, can be tuned for therapeutic value. Despite inducing small levels of stress, they ultimately benefit the host cell, potentially for therapeutic applications. As an analogous example, we have shown that pharmacologically increasing levels of reactive metabolites can also activate NRF2, indicating there may be alternative, and perhaps superior, mechanisms for activating NRF2 therapeutically.
What specific applications do you imagine?
Ultimately, we believe that HPPE and molecules like it may have potential therapeutic value even though BACH1 is not the direct target. HPPE and other related molecules like ASP8731, a molecule that underwent Phase 1 testing that is derived from the HPPE chemical scaffold, show interesting efficacy in preclinical models to treat neurodegenerative and autoimmune diseases.
What part of your work was the most challenging?
Trying to confirm the specificity of a small molecule to BACH1 is challenging given that the speculated binding area is an intrinsically disordered region of the protein. Because a complete structure is not available for analysis, we needed to perform experiments that could “rule out” alternative targets.
A key challenge in the future will be to identify validated direct pharmacological ligands to BACH1, so to discover and confirm specific drugs or molecules that can directly bind to and and inhibit BACH1, because, as we posit here, such molecules do not exist in the public domain.
Anything else you would like to add?
We would like to thank the individuals who laid the basis for this discovery, including the labs of Kauhiko Igarashi and others.
Thank you for these insights.
The paper they talked about:
- HPPE Activates NRF2 Signaling by Liberating Heavy Metal Stores,
Rebecca Freeman, Michael Bollong,
ChemBioChem 2024.
https://doi.org/10.1002/cbic.202400529
Michael J. Bollong is an Associate Professor in the Department of Chemistry at The Scripps Research Institute, La Jolla, CA, USA.
Rebecca Freeman is a Ph.D. student at Scripps Research Institute.