Kevin Bernot, INSA Rennes, Université de Rennes, France, Yann Sarrazin, Université de Rennes, and colleagues have discovered that although the prolate single molecule magnets known from the textbook have large ground state splittings, this does not lead to strong magnetism.
What did you do?
We are coordination chemists interested in building magnetic molecules. Those molecules, if well isolated from their surroundings, can act as magnets at the molecular level and are then called Single-Molecule Magnets (SMM). They are being considered for nanoscale information storage and quantum applications.
Lanthanides are the best ions for constructing SMMs due to their large magnetic moment and magnetic anisotropy. They are categorized into oblate ions (Ce³⁺, Pr³⁺, Nd³⁺, Tb³⁺, Dy³⁺, Ho³⁺) and prolate ions (Pm³⁺, Sm³⁺, Er³⁺, Tm³⁺, Yb³⁺), depending on the shape of their free ion electronic density.
Oblate ions have an electron density distribution that is flattened at the poles, resembling a disc shape. This shape is stabilized when the ions are axially coordinated with ligands (molecules or ions that donate electron pairs). Oblate ions are widely used because such axial coordination is accessible with high coordination numbers preferred by lanthanides. On the contrary, prolate ions have an electron density distribution that is elongated along one axis, resembling a rugby ball or an ellipse. This shape is stabilized by an equatorial coordination that is way more difficult to achieve with lanthanides.
In this paper, however, we focus on the less-studied prolate ions, specifically Yb³⁺, with the aim of obtaining the best-performing prolate SMM. Previous theoretical studies [1] postulated that a fictive neutral and tris-coordinated Yb³⁺ triangular planar molecule would show the best possible electronic structure for a prolate ion, potentially leading to extremely relevant SMM behavior.
With this in mind, we make this theoretical dream come true by synthesizing the expected molecule, digging into already known compounds, as well as investigating various derivatives for comparison. We confirm the exceptional electronic structure of the synthesized molecule; however, we also show that its magnetic performance is much weaker than expected.
We hypothesize that the anomalies observed in its infrared emission spectra could be an experimental signature of active vibronic contributions in the molecule, which may detrimentally affect its magnetic behavior. In doing so, we experimentally demonstrate that the stiffness of the molecule and its phonon coupling with the surroundings are key to achieving high-performing SMMs.
Why are you interested in this?
Research in the field of SMMs is very active, and molecules with spectacular magnetic behavior have been observed. However, the performance of SMMs is often limited by vibronic or spin-phonon coupling phenomena, which are difficult to disentangle from other relaxation phenomena and challenging to rationalize. This complexity makes efforts to enhance the performance of SMMs for practical applications difficult.
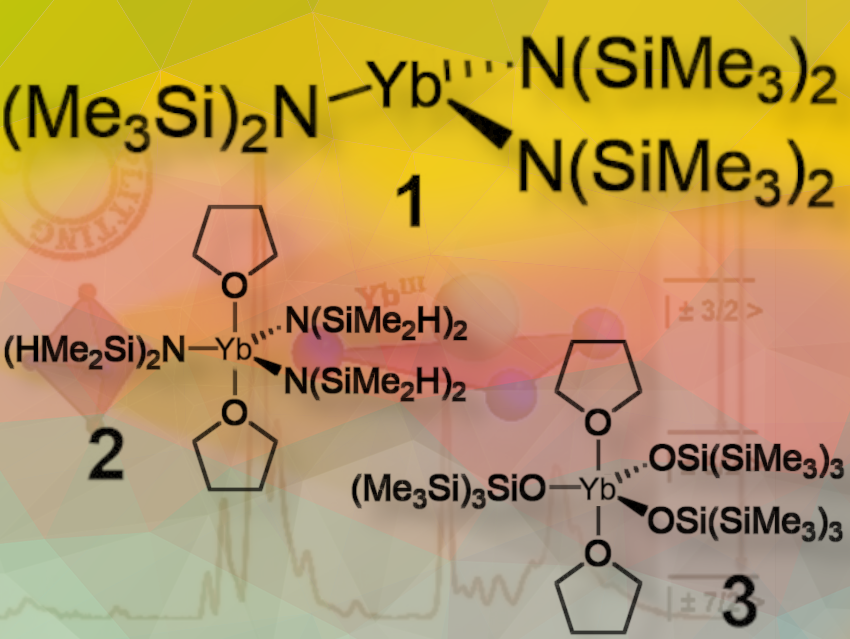
By working with an ideally symmetric compound such as Yb{N(SiMe₃)₂}₃ (1), where three amides occupy the equatorial positions around the central cation, we can induce an optimal electronic structure for prolate Yb³⁺ with an MJ energy splitting. MJ refers to the differences in energy levels associated with the magnetic quantum states of the system, which are critical for understanding the material’s magnetic behavior. A larger MJ splitting generally indicates better performance in magnetic applications, particularly in SMMs.
Indeed, when comparing ideally symmetric compound with other five-coordinate Yb³⁺ compounds that have a trigonal bipyramidal geometry: Yb{N(SiMe₂H)₂}₃·(thf)₂ (2) and Yb{OSi(SiMe₃)₃}₃·(thf)₂ (3), a significant alteration in the electronic structure is observed, as theoretically predicted. This implies that the arrangement of atoms and the resulting symmetry can greatly influence the electronic and magnetic properties of the material.
Thus, we demonstrate that working with symmetrical molecules is essential for understanding their optimal electronic structures and the resulting magnetic behavior. This understanding will enhance our knowledge of the mechanisms that govern their magnetic properties and enable us to optimize them further.
What is new and cool about your findings?
The coolest thing was certainly the ability to synthesize a molecule predicted in 2021 to be the best prolate SMM. This is a very interesting example of science where experimental data supports independent in silico predictions!
However, to do this, we had to design extremely unusual and unstable compounds, as tris-coordinated lanthanides are quite rare. Obtaining large enough quantities of crystalline material to carry out reliable magnetic and luminescent measurements on molecules that are so sensitive to air, water, temperature, etc. was a real challenge. We also had to adapt all our instruments and processes. In addition, repeatability was essential to guarantee the quality of our results.
But even cooler is that the trigonal symmetry acts like a fantastic zoom lens on the infra-red emission spectra, allowing us to see details that we wouldn’t normally see. We look forward to extending our study to other compounds and learning more about the appealing peak substructures we have observed. At this stage, we only have hypotheses that we hope to confirm soon.
Last, and on a more chemical aspect, it was nice to work with Yb³⁺, which, despite its paramagnetic nature and having one unpaired f-electron, could still be analyzed by NMR spectroscopy, allowing for very efficient characterization.
What are your key findings?
We demonstrate on real molecules, not only on fictitious ones, that the trigonal planar geometry is the best for optimizing the MJ splitting of the Yb³⁺ ion. This splitting is key for observing both magnetic properties (particularly SMM behavior) and luminescent properties. Indeed, in 2012, some of us demonstrated that luminescence could help characterize the magnetic behavior of SMMs [2]. Today, however, the opposite is true, as the search for best optimized SMMs could lead to new findings in the field of lanthanide luminescence.
Our findings go far beyond the field of SMMs, as to the best of our knowledge, no such MJ splitting has been observed before. More precisely, the infrared emission spectra of Yb³⁺ ions generally range from 200 to 400 cm⁻¹, which is already significant. In contrast, for compound (1), emission peaks span over 1312 cm⁻¹! This allows us to see unprecedented optical signatures, and we hope that spectroscopists and specialists in the field will be drawn to study such symmetrical compounds and explain the anomalous peak substructures we observe.
Do you imagine specific applications?
Our research is fundamental in nature, and we mainly build knowledge rather than devices. But lanthanide-based molecular materials offer immense prospects in nanometric magnetic data storage, luminescent devices, and quantum applications.
Above all, it is spectacular to see how SMM research brings together scientists from extremely diverse fields, ranging from pure synthetic chemists to spectroscopists and quantum physicists. And very often, interesting things emerge from these cross-fertilizations.
What part of your work was the most challenging?
The handling and preparation of the compounds for luminescence and magnetic measurements were the trickiest parts of the work. These metal complexes are very sensitive to air and humidity, and they tend to decompose slowly during storage, even when properly sealed. For example, compound (1) was isolated as lemon-yellow crystals on several occasions, but it gradually lost its coloration while stored in an inert atmosphere at room temperature.
Therefore, we had to reconsider all our processes, synchronize the magnetic and luminescent experiments, and, above all, repeat them multiple times! We carried out many measurement campaigns before obtaining a robust and repeatable set of data on pure compounds.
This has been a steep learning curve, and we are glad the story is now out there. However, this is just the beginning of our journey, and much more is still to come!
Thank you very much for these insights.
The paper they talked about:
- Luminescence and Single-Molecule Magnet Properties in Ideal Symmetry Compounds: Example of a Near-Planar Tricoordinate Ytterbium(III) Amide,
Nimisha Jain, Félix Houard, Rémi Marchal, Marie Cordier, Boris Le Guennic, Yan Suffren, Yann Sarazin, Kevin Bernot,
ChemistryEurope 2024.
https://doi.org/10.1002/ceur.202400062
Kevin Bernot is a Professor at the INSA Rennes, Université de Rennes in France.
Yann Sarrazin is CNRS researcher at the Université de Rennes, France.
References
[1] Martín Amoza, Silvia Gómez-Coca, Eliseo Ruiz, Magnetic anisotropy in YbIII complex candidates for molecular qubits: a theoretical analysis, Phys. Chem. Chem. Phys 2021, 23, 1976–1983. https://doi.org/10.1039/D0CP05422D
[2] Giuseppe Cucinotta, Mauro Perfetti, Javier Luzon, Mael Etienne, Pierre-Emmanuel Car, Andrea Caneschi, Guillaume Calvez, Kevin Bernot, Roberta Sessoli, Magnetic Anisotropy in a Dysprosium/DOTA Single-Molecule Magnet: Beyond Simple Magneto-Structural Correlations, Angew. Chem. Int. Ed. 2012, 51, 1606–1610. https://doi.org/10.1002/anie.201107453