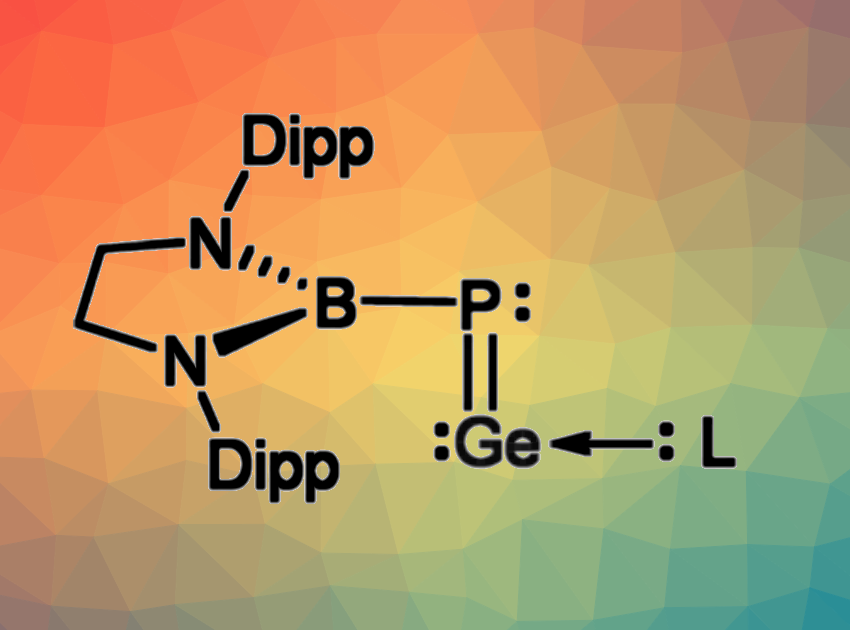Phosphagermylenylidenes (R–P=Ge) can be considered heavier analogs of isonitriles (R–N=C). Due to the reduced tendency of the heavier elements to form double bonds, the synthesis of such species can be be challenging. Phosphagermylenylidenes had not been isolated so far, neither in free form nor in complexes with a Lewis base.
Liu Leo Liu, Southern University of Science and Technology, Shenzhen, China, and Nankai University, Tianjin, China, have synthesized a stable monomeric phosphagermylenylidene, stabilized by a Lewis base (pictured, Dipp = 2,6-iPr2C6H3, L = a cyclic (alkyl)(amino)carbene). In addition to the cyclic (alkyl)(amino)carbene (CAAC) as a Lewis base, the team incorporated sterically demanding N-heterocyclic boryl (NHB) unit to stabilize the product. They started from a lithium NHB-phosphide, which was reacted with GeCl2(dioxane) in tetrahydrofuran (THF). This gave a dimeric species with a central P2Ge2 unit.
This intermediate was then converted to the desired monomer by adding the CAAC. According to the researchers, the resulting species represents the first instance of an isolable phosphorus/germanium analog of isonitrile.
- Carbene-Stabilized Phosphagermylenylidene: A Heavier Analog of Isonitrile,
Jiancheng Li, Xin-Feng Wang, Chaopeng Hu, Liu Leo Liu,
J. Am. Chem. Soc. 2024.
https://doi.org//10.1021/jacs.4c04434