Researchers have found a way to produce olefins with a chain length of more than twelve carbon atoms directly from carbon monoxide and water. The new process, introduced by Hongliang Li, University of Science and Technology of China, Hefei, Jie Zheng, University of Science and Technology of China and Anhui University of Technology, Ma’anshan, China, and colleagues, takes a decades-old variant of the Fischer–Tropsch synthesis and repurposes it by adding a specific catalyst and a phase-transfer agent. It also provides an opportunity to use more environmentally friendly sources for heavy olefin production [1].
Products of the Fischer–Tropsch Synthesis
The Fischer–Tropsch reaction, the direct hydrogenation of carbon monoxide, has been used for over a century to produce a wide variety of hydrocarbons, depending on which catalyst is used. Roughly speaking, of these catalysts, iron- and cobalt-based catalysts tend to produce light, short-chain, aromatic, and olefinic hydrocarbons, while ruthenium produces heavier, fully saturated products.
Looking at these products, it is clear that there is a gap where the fraction containing long-chain olefinic hydrocarbons should be. Olefins with chain lengths greater than twelve can be synthesized in other processes, either bottom-up by oligomerization–dehydrogenation routes using ethylene as the starting reagent, or top-down by cracking waxes. However, these processes require multiple steps or are energy-intensive, not to mention they rely on fossil sources. To find a more sustainable alternative, the team re-investigated the Fischer–Tropsch reaction, turning their attention to the starting reactants.
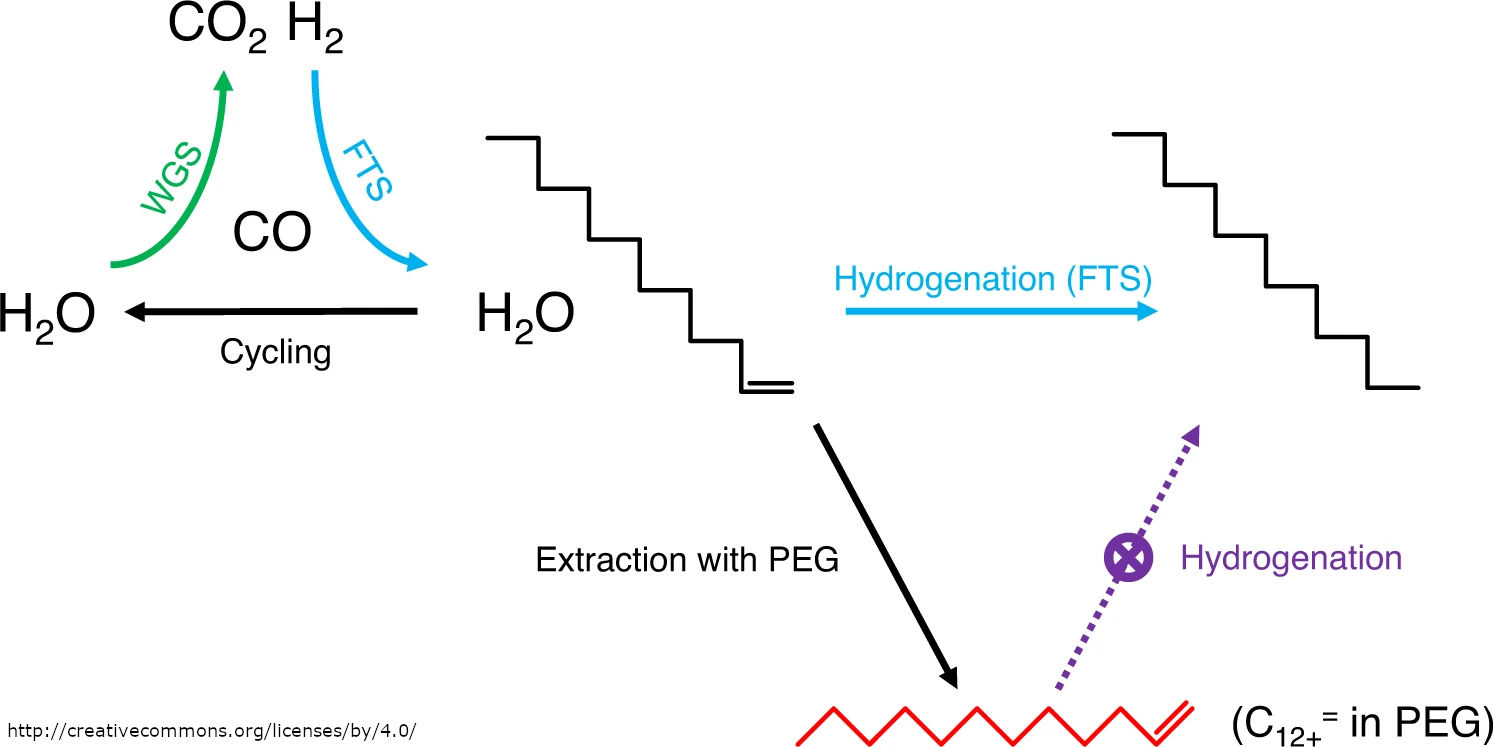
They found inspiration in the decades-old work of German chemists Herbert Kölbel and Friedrich Engelhardt. In the 1950s, Kölbel and Engelhardt introduced a variation on Fischer–Tropsch synthesis (FTS) to avoid the need for an external hydrogen source. Instead of supplying this gas from outside, hydrogen was generated in situ using the water gas shift (WGS) reaction, the generation of carbon dioxide and hydrogen from carbon monoxide and water. By coupling this reaction with the Fischer–Tropsch process in a cascade, the Kölbel–Engelhardt synthesis was successful for cases where hydrogen supply was limited.
The Kölbel–Engelhardt synthesis is not widely used today and has not been further developed. However, it could be helpful if better control of the kinetics of the hydrogenation reaction is desired. The team used this cascade process to suppress the hydrogenation capability of the ruthenium catalyst in the Fischer–Tropsch reaction while retaining its carbon-coupling activity, allowing longer chains to be formed.
Finding the Right Catalyst Mix
Cascade reactions, however, often require different catalysts for each reaction. The Fischer–Tropsch synthesis benefits from ruthenium, while the water gas shift reaction favors molybdenum-based materials. To solve this, the team mixed platinum nanoparticles on a molybdenum nitride support with ruthenium, achieving maximum efficiency. In the water gas shift reaction, molybdenum nitride catalyzes the dissociation of water, with the platinum nanoparticles transferring the oxygen to carbon monoxide, and in the Fischer–Tropsch synthesis, ruthenium excels at carbon coupling and hydrogenation.
A phase-transfer agent was also added. To prevent overhydrogenation to paraffins, the generated olefins had to be separated from the catalyst. Using polyethylene glycol (PEG) as a simple and effective phase-transfer agent to stabilize the olefins on the catalyst surface, the researchers reported almost complete extraction of 1-dodecene, a product representative of the extra-heavy olefins generated.
To make the process greener, the team also investigated a mixture of two less expensive, non-noble catalyst combinations. Copper, zinc oxide, and aluminum oxide for the water gas shift reaction, together with cobalt and aluminum oxide for the Fischer–Tropsch synthesis, also gave long-chain olefins. Although yields and selectivity were lower than in the original set-up, the researchers concluded that the strategy can be applied universally, with the option of fine-tuning the selectivity of olefins to paraffins.
At moderate pressures of 2–4 MPa and temperatures of up to 200 °C, the researchers reported conversions of carbon monoxide to long-chain olefins in a process that is less complex and energy-intensive than other available processes. In addition, because carbon monoxide can be derived from carbon dioxide, the process can be carried out sustainably instead of using established fossil-based sources.
Reference
[1] Chuanhao Wang, Junjie Du, Lin Zeng, Zhongling Li, Yizhou Dai, Xu Li, Zijun Peng, Wenlong Wu, Hongliang Li, Jie Zeng, Direct synthesis of extra-heavy olefins from carbon monoxide and water, Nat. Commun. 2023. https://doi.org/10.1038/s41467-023-37599-2