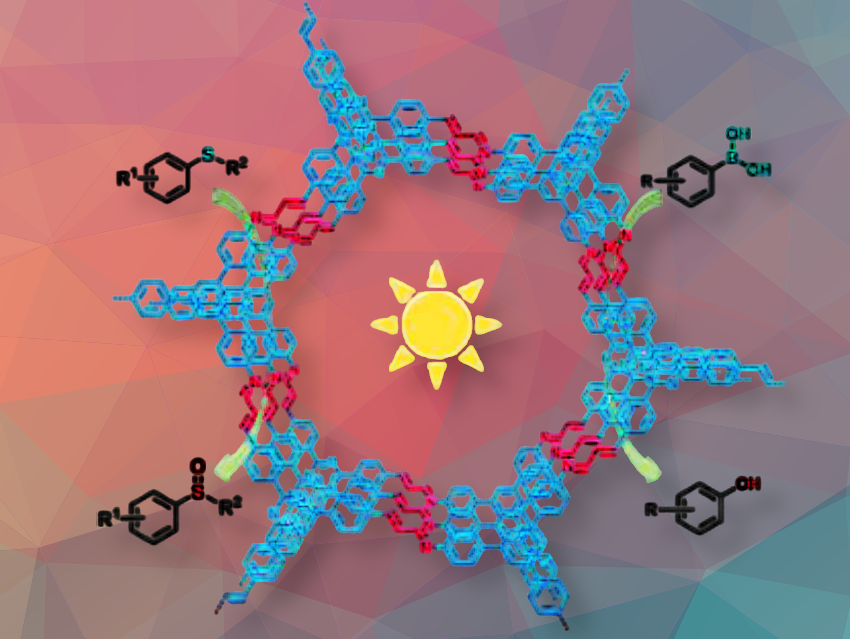
Xiao Xiao, Jilin Medical University, P.R. China, Xiaoming Liu, Jilin University, Changchun, P.R. China, and colleagues have synthesized two azadiene-linked covalent organic frameworks (COFs), COF-JLU236 (pictured) and COF-JLU237. The team synthesized COF-JLU236 and COF-JLU237 via reversible imine condensation of 3,3′,3”-((1,3,5-triazine-2,4,6-triyl)tris(benzene-4,1-diyl))triacrylaldehyde (TTTB) with 1,3,5-tris-(4-aminophenyl)benzene (TAPB) or 1,3,5-tris-(4-aminophenyl)triazine (TAPT) under solvothermal conditions (100 °C, 3 days) in dioxane/mesitylene and aqueous acetic acid, yielding crystalline COFs with 97% and 91% yields, respectively. The precursor TTTB was obtained through Pd-catalyzed coupling.
These new materials have high crystallinity and large surface areas, featuring an extended π-conjugated azadiene linkage designed as a proof of concept. This linkage broadens the absorption range and suppresses exciton recombination, thereby enhancing photocatalytic efficiency.
COF-JLU236 demonstrated excellent photocatalytic activity for the selective oxidation of sulfides to sulfoxides and arylboronic acids to phenols under visible light using O₂ as the green oxidant. Optimal performance was achieved in methanol with high yields (up to 97%) and substrate tolerance; mechanistic studies, including EPR and quenching experiments, revealed that O₂•⁻ and ¹O₂ species play key roles, with O₂•⁻ being predominant. The material showed high recyclability over multiple cycles without loss of activity, and large-scale reactions confirmed its potential for practical applications.
- Azadiene-Linked Carbon-Organic Frameworks for Enhanced Photocatalytic Efficiency in Organic Synthesis,
Xiaoming Liu, Yuxin Hou, Yuchen Jin, Shanshan Zhu, Xiao Xiao, Huijuan Yue,
Chem. Eur. J. 2025.
https://doi.org/10.1002/chem.202500934