In contrast to five-membered phosphorus heterocycles, six-membered phosphorus heterocycles have received comparatively less attention, largely due to the absence of versatile and convenient synthetic methods. Although some six-membered cyclic phosphines and their derivatives have emerged recently, the chemistry of λ⁵-phosphinines—six-membered phosphacycles containing a phosphorus ylide—remains in its early stages.
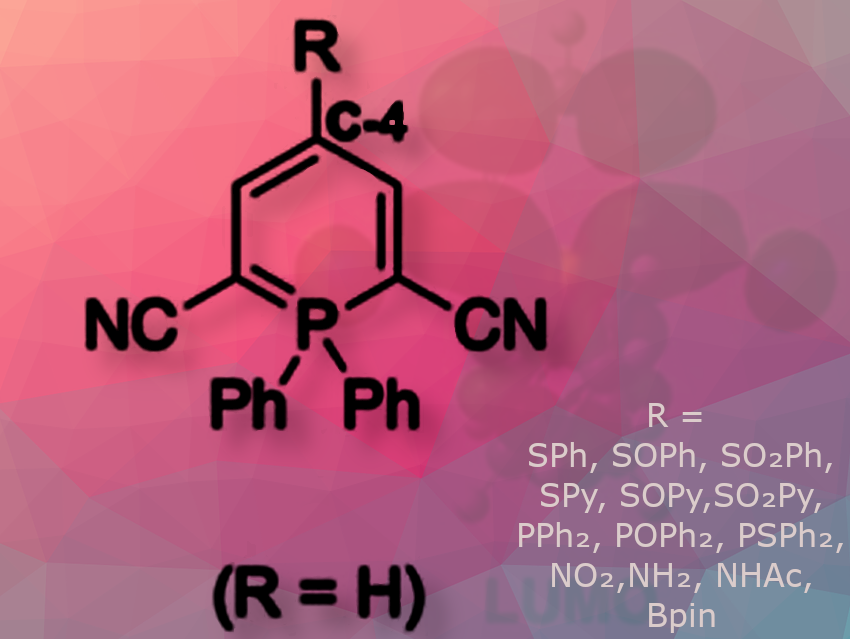
Minoru Hayashi and colleagues, Ehime University, Matsuyama, Japan, have synthesized a new fluorophore, 2,6-dicyano-λ⁵-phosphinine (pictured), developed multiple derivatization strategies, and prepared λ⁵-phosphinines bearing S-, P-, N-, and B-substituents at the C-4 position.
λ⁵-Phosphinines have a unique distribution of frontier molecular orbitals: the C-4 position contributes significantly to the highest occupied molecular orbital (HOMO), but not to the lowest unoccupied molecular orbital (LUMO). Consequently, substitution at this position can selectively modulate the HOMO energy without affecting the LUMO, enabling precise control over photophysical properties via the electronic nature of the substituent.
The heteroatom-substituted phosphinines showed strong fluorescence with high quantum yields, except for the nitro derivative. Electron-donating groups caused red shifts, while electron-withdrawing groups induced blue shifts in absorption and fluorescence. Notably, sulfoxide derivatives showed unexpectedly bright emission, making them promising candidates for oxidant detection. P-substituted phosphinines showed emission independent of oxidation state, while N- and B-substituted variants demonstrated fluorescence changes upon imine formation or anion complexation, enabling selective chemical sensing.
The team also showed that their synthesized heteroatom-substituted phosphinines can be applied to selective and qualitative detection of several chemicals. Redox, complexation, and the reaction of the functional group cause changing their fluorescence.
- Heteroatom-Functionalized λ5-Phosphinines—Synthesis and Properties,
Minoru Hayashi, Makoto Yano, Dam Thi Huyen Trang, Ryusei Ikeda, Hidetoshi Ohta,
Eur. J. Org. Chem. 2025.
https://doi.org/10.1002/ejoc.202500256