Fuyuhiko Inagakia and Ryo Murakami, Kobe Gakuin University (Kobe Gakuin Daigaku), Minatojima, Japan, discuss a recently published study in which they, together with colleagues, demonstrate that CO₂ can be spontaneously released from aralkylamine–CO₂ complexes at room temperature, eliminating the need for external energy input and offering a promising advance in CO₂ capture technology.
What did you do?
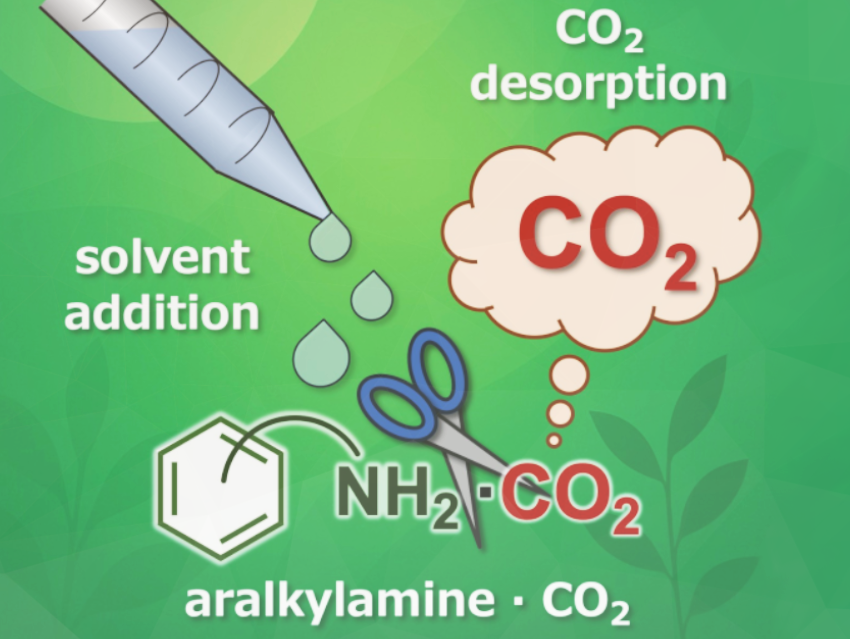
We developed a new strategy for CO₂ capture and release based on a solvent-triggered retro-neutralization reaction using aralkylamines. Remarkably, this system enables the release of CO₂ at room temperature by simply adding a solvent—no external energy input such as heat, pressure, light, or electricity is required.
Why are you doing this?
Conventional CO₂ capture technologies, while effective, are hindered by high energy demands during the CO₂ desorption phase. Our objective was to overcome this limitation by creating a low-energy, sustainable method that simplifies the CO₂ separation process and enhances the overall feasibility of carbon capture applications.
How is your process working?
The process works via solubility-driven retro-neutralization: when a suitable solvent is added to an aralkylamine–CO₂ complex, the solvent disrupts the ionic interactions between the amine and CO₂, shifting the equilibrium back toward free amine and gaseous CO₂.
What is new and cool about your work?
What sets our work apart is the discovery that CO₂ can be released from CO₂ absorbed aralkylamines at room temperature simply by adding a solvent, without changing the chemical structure of the amine or applying any external energy. This overturns the conventional assumption that CO₂ desorption from amines is necessarily external energy intensive.
Moreover, we found that the CO₂ release does not correlate with the boiling point or polarity of the solvent—highlighting that solubility, rather than volatility, drives the desorption. The system leverages the unique self-assembling properties of aralkylamines, forming supramolecular structures that can be selectively disrupted by solvent addition to trigger CO₂ release.
What are your key findings?
Our key findings include:
- Selective CO₂ capture from ambient air using aralkylamines, with minimal water uptake, thanks to the hydrophobic environment created by reverse amphiphilic self-assembly.
- Room-temperature CO₂ desorption via solvent addition, achieving up to 79% CO₂ release even in a closed system.
- Demonstrated reusability of the amine solvent system over ten consecutive cycles without significant degradation, as confirmed by NMR analysis.
- Structural elucidation by X-ray crystallography revealed a pillar-like architecture stabilized by π–π and ionic interactions, which disassemble upon solvent addition, facilitating CO₂ release through a retro-neutralization mechanism.
- Introduction of the concept of “solvent-swing” desorption, expanding the toolbox of CO₂ capture beyond energy-based methods.
What specific applications do you imagine?
This technology is especially well-suited for Direct Air Capture (DAC) systems, where energy efficiency and simplicity are key for scalability. The low-temperature, low-energy nature of this process makes it highly attractive for use in remote areas, decentralized carbon recycling units, or integration into circular chemical processes.
In the long term, we envision a platform technology for external energy-free gas separation, which could be extended beyond CO₂ to other gases or ionic species, with applications in environmental remediation, chemical purification, and green manufacturing.
What part of your work was the most challenging?
One of the most technically challenging aspects was proving the CO₂ release mechanism from CO₂-absorbed aralkylamines without external energy input and distinguishing it from physical desorption or degradation. It required extensive control experiments, quantitative closed-system testing, and solid-state structural analyses to support the solubility-based retro-neutralization model.
Additionally, optimizing the experimental conditions (e.g., gas flow rates, solvent types, and moisture interference) to ensure reproducibility across open and closed systems was complex and time-intensive.
Thank you very much for sharing these insights.
The paper they talked about:
- Solvent-Swing CO₂ Capture with Aralkylamines,
Ryo Murakami, Hiroto Tanishima, Risa Otsuka, Ayaka Uchida, Narumi Iwamoto, Fuyuhiko Inagaki,
ChemSusChem 2025.
https://doi.org/10.1002/cssc.202500112
Fuyuhiko Inagaki is a Full Professor in the Faculty of Pharmaceutical Sciences at Kobe Gakuin University (Kobe Gakuin Daigaku) in Minatojima, Japan.
Ryo Murakami is an Assistant Professor in the Faculty of Pharmaceutical Sciences at Kobe Gakuin University (Kobe Gakuin Daigaku) in Minatojima, Japan.


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)

