Fabian Reiß and Torsten Beweries, Leibniz Institute for Catalysis, Rostock, Germany, and colleagues have synthesized the dinuclear compound [(Cp₂Zr)₂(µ-Me)(µ-C₂Ph)]. The compound has unique and unusual structural properties, including a bridging methyl group that is highly “fluxional,” meaning it can easily change or move within the compound. This fluxional methyl group allows the compound to show a wide variety of uncommon chemical behaviors or reactions, making it interesting for further study and potential applications.
The team first synthesized LiC₂Ph by reacting phenylacetylene with n-BuLi in diethyl ether, followed by washing with n-hexane. This precursor then reacts with [Cp₂Zr(Me)Cl] in toluene to form a mononuclear complex in high yield. The resulting complex is subsequently reacted with Rosenthal’s zirconocene(II) source, [Cp₂Zr(py)(µ²-Me₃SiC₂SiMe₃)] (py = pyridine), to produce the dinuclear Zr alkynyl methyl complex [(Cp₂Zr)₂(µ-Me)(µ-C₂Ph)], isolated as an orange solid with up to 77% yield.
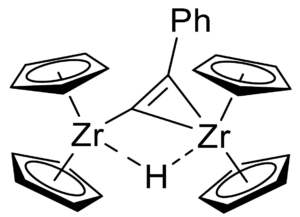 [(Cp₂Zr)₂(µ-Me)(µ-C₂Ph)] shows catalytic activity for the dehydrocoupling of amine boranes, with a dinuclear hydride-bridged alkynyl complex (pictured on the right) being formed as a catalytically relevant species.
[(Cp₂Zr)₂(µ-Me)(µ-C₂Ph)] shows catalytic activity for the dehydrocoupling of amine boranes, with a dinuclear hydride-bridged alkynyl complex (pictured on the right) being formed as a catalytically relevant species.
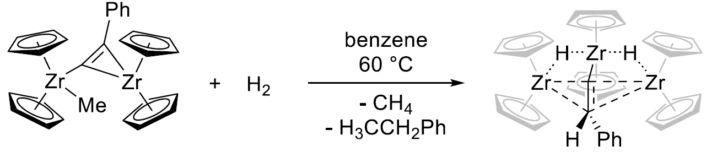
When reacted with hydrogen, [(Cp₂Zr)₂(µ-Me)(µ-C₂Ph)] undergoes hydrogenation of the alkynyl ligand, producing a highly labile trinuclear hydride-bridged complex (pictured below), as a possible intermediate of zirconocene dihydride/ethyl benzene formation. This intermediate has an unusual, distorted planar tetracoordinate environment at the central carbon atom, which is located between the three Zr centers.
The reaction of [(Cp₂Zr)₂(µ-Me)(µ-C₂Ph)] with 2-cyanopyridine and acetonitrile results in substrate reduction, with no further coupling observed.
The reactivity of [(Cp₂Zr)₂(µ-Me)(µ-C₂Ph)] demonstrates the ability of zirconocenes to stabilize rare bond structures. The researchers confirmed this by spectroscopy, structural analysis, and computational methods. According to them, their findings lay the groundwork for future use of these mixed-valent homo- and heterobimetallic early transition metal complexes in stoichiometric and catalytic bond activation reactions.
- The Dinuclear Zirconocene Complex [(Cp₂Zr)₂(µ-Me)(µ-C₂Ph)] as a Platform for Small Molecule Activation,
Hanan Al Hamwi, Mirko Rippke, Kevin Lindenau, Anke Spannenberg, Martin Lamac, Fabian Reiß, Torsten Beweries,
Chem. Eur. J. 2025.
https://doi.org/10.1002/chem.202500857
![Unique Features of the Dinuclear Zirconocene Complex [(Cp₂Zr)₂(µ-Me)(µ-C₂Ph)]](https://www.chemistryviews.org/wp-content/uploads/2025/03/202503_Dinuclear-Zirconocene-Complex.png)