Methane has a symmetrical tetrahedral structure with nonpolar C–H bonds. This lack of polarity makes it unreactive toward many catalysts, as it does not readily interact with polar reagents or transition metal complexes that facilitate bond activation. The C–H bond in methane has a high bond strength.
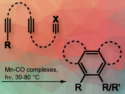
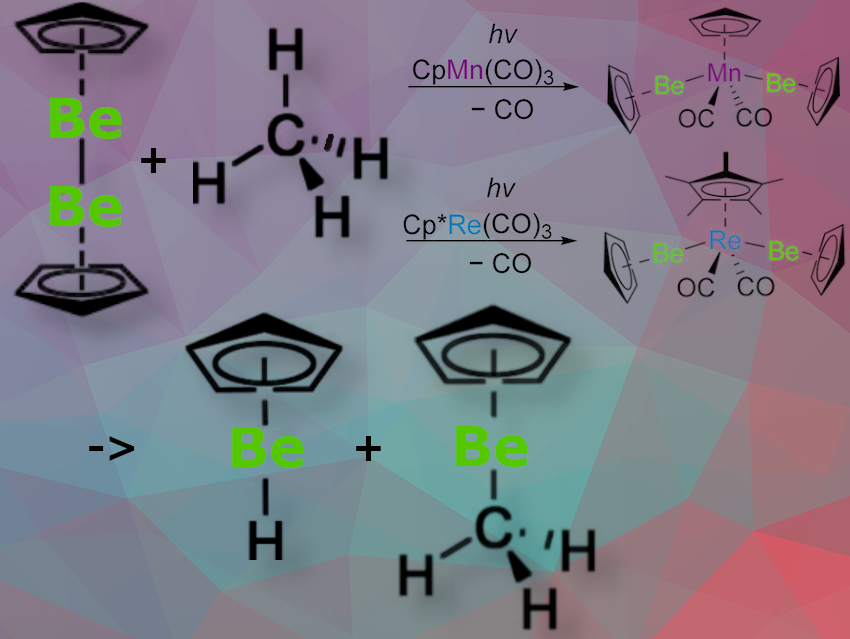
Josef T. Boronski, Imperial College London, UK, Simon Aldridge, University of Oxford, UK, and colleagues have developed a catalytic method to transform methane and benzene C–H bonds into C–Be and H–Be bonds using CpBeBeCp. The reaction occurs under photochemical conditions and at atmospheric pressure, with CpMn(CO)₃ or Cp*Re(CO)₃ serving as catalysts.
The team successfully isolated the proposed intermediates in these beryllation reactions, trans-CpMn(CO)2(BeCp)2 and trans-Cp*Re(CO)2(BeCp)2, )₂—both of which are stable, colorless, trivalent, and 18-electron diamagnetic complexes.
Using quantum chemical calculations, the team compared methane beryllation with borylation by CpMn(CO)₃. The results suggest that the strong σ-donating ability of beryllyl ligands and their highly Lewis acidic beryllium centers make methane functionalization possible—something that boron analogues cannot achieve.
The researchers believe their findings could guide the design of future homogeneous catalytic systems for C–H elementation.
- Methane Beryllation Catalyzed by a Base Metal Complex,
Josef T. Boronski, Agamemnon E. Crumpton, Job J. C. Struijs, Simon Aldridge,
J. Am. Chem. Soc. 2025.
https://doi.org/10.1021/jacs.5c02179

![Unique Features of the Dinuclear Zirconocene Complex [(Cp₂Zr)₂(µ-Me)(µ-C₂Ph)]](https://www.chemistryviews.org/wp-content/uploads/2025/03/202503_Dinuclear-Zirconocene-Complex-125x94.png)