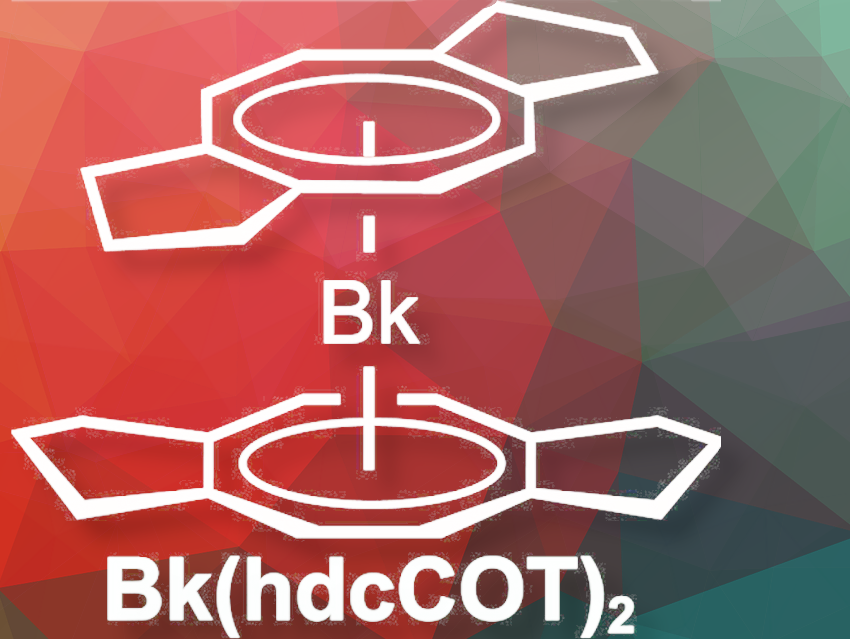
Dominic R. Russo, Lawrence Berkeley National Laboratory, Berkeley, and University of California, Berkeley, CA, USA, and colleagues have synthesized and structurally characterized the berkelocene Bk(hdcCOT)₂ (hdcCOT = hexahydrodicyclopenta[8]annulene), where the synthetic element berkelium is sandwiched between two cyclooctatetraene-based ligands (pictured above). The team prepared the organometallic complex from 0.3 mg of berkelium-249 (t1/2 = 330(4) days), the most readily available isotope of this scarce and radioactive element. For example, the entire experimental process, had to be achieved within ~48-hours.
Anhydrous BkCl₃(DME)ₙ (DME = 1,2-dimethoxyethane) was synthesized by reacting hydrated BkCl₃ with Me₃SiCl in DME, yielding an off-white solid. This precursor was suspended in tetrahydrofuran (THF), and K₂hdcCOT was added, forming a yellow-orange solution. After 16 hours of stirring, Ph₃CCl was introduced, immediately turning the solution indigo. Following work-up and evaporation, indigo-colored crystals were obtained and confirmed as Bk(hdcCOT)₂ via X-ray diffraction.
Single-crystal x-ray diffraction shows a tetravalent berkelium ion between two substituted cyclooctatetraene ligands, resulting in the formation of berkelium–carbon bonds. The coordination in berkelocene resembles that of uranocene, and calculations show that the berkelium 5f orbitals engage in covalent overlap with the δ-symmetry orbitals of the cyclooctatetraenide ligand π system. Reduced charge transfer from the ligands, compared to uranocene and other actinocenes, enhances the stability of tetravalent berkelium by maximizing contributions from its half-filled Bk4+ 5f7 configuration.
The sandwich structure ferrocene, Fe(Cp)₂ (Cp = C5H5), was synthesized in the early 1950s by Wilkinson, Fischer, and colleagues. The first Cp complexes of actinides were highly reactive and air-sensitive, while actinide alkyl and carbonyl complexes—ubiquitous among d-block metals—were far too unstable to isolate. In the late 1960s, uranocene, U(COT)₂ (COT = C8H8), was synthesized by Streitwieser, Raymond and colleagues. It shoed remarkable thermodynamic stability compared with actinide Cp complexes, attributed to the COT ligands being a better match for the U 5f orbitals in terms of size, energy, and symmetry. By 1976, actinocenes from Th to Pu had been characterized, along with cerocene. Berkelium (Bk) was discovered by Glenn T. Seaborg and colleagues in 1950.
- Berkelium–carbon bonding in a tetravalent berkelocene,
Dominic R. Russo, Alyssa N. Gaiser, Amy N. Price, Dumitru-Claudiu Sergentu, Jennifer N. Wacker, Nicholas Katzer, Appie A. Peterson, Jacob A. Branson, Xiaojuan Yu, Sheridon N. Kelly, Erik T. Ouellette, John Arnold, Jeffrey R. Long, Wayne W. Lukens Jr, Simon J. Teat, Rebecca J. Abergel, Polly L. Arnold, Jochen Autschbach, Stefan G. Minasian,
Science 2025.
https://doi.org/10.1126/science.adr3346