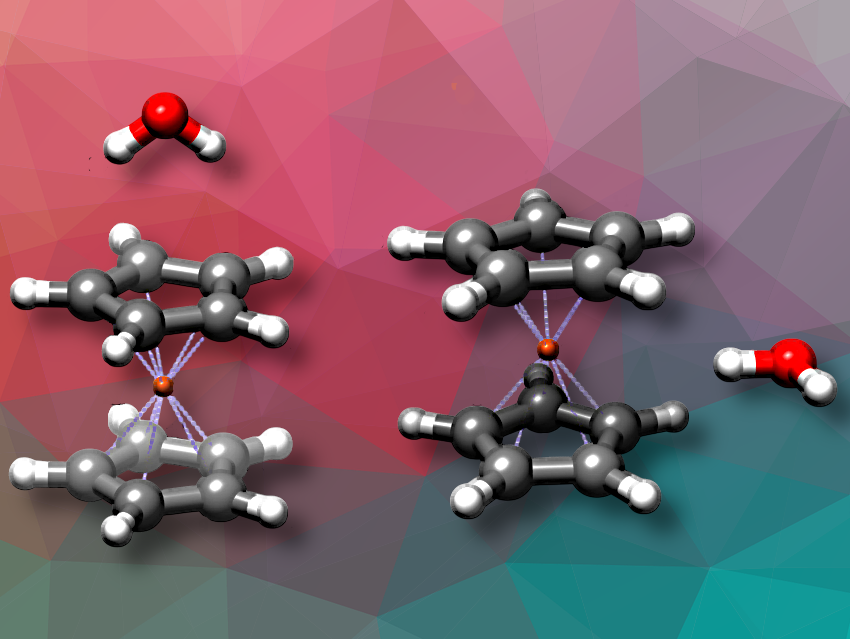
Juan Carlos Lopez, Universidad de Valladolid, Spain, and colleagues have characterized the structure of the ferrocene-water ((Cp)2Fe:H2O) complex using rotational spectroscopy and computational chemistry. The team found that this complex exists in two almost isoenergetic species, where water interacts either axially with the exo Cp π cloud or equatorially pointing to the iron atom. Water rotates almost freely within the complex. The quantum mechanical effects of water rotational dynamics prevent the experimental location of the water hydrogen atoms.
Both arrangements lead to binding energies above -11 kJ/mol, which are notably strong for a neutral complex of this nature. In both cases, experimental and theoretical data reveal minor deformations in the ferrocene structure caused by its interaction with water.
As ferrocene lacks a permanent dipole, forming a complex with water enables its structural characterization via rotational spectroscopy and provides insights into its proton affinity, an important property in organometallic chemistry.
Understanding these dynamical effects provides a more complete picture of hydrogen bonding and intermolecular interactions in ferrocene systems, contributing to broader research on organometallic solvation, solubility, and reactivity, the researchers say. Insights into the redistribution of electron density also underline the importance of such studies for fundamental chemistry and potential applications in catalysis and materials science, they say.
- Unveiling the Dual Nature of the Smallest Aqueous Ferrocene Complex,
Susana Blanco, Andrés Verde, Juan Carlos Lopez, Manuel Yañez, Maria de la Merced Montero Campillo, Ibon Alkorta,
Chem. Eur. J. 2025.
https://doi.org/10.1002/chem.202500128