Metal complexes bearing boryl (BX2–) ligands can be useful in organic synthesis, e.g., for the C–H functionalization/borylation of unactivated alkane or arene substrates. Metal complexes with the heavier group 13 analogues of boryl ligands (i.e., aluminyl, gallyl, indyl ligands, etc.) are interesting research targets. Investigating these species could provide fundamental information about trielyl ligands, and the complexes might enable new reactions.
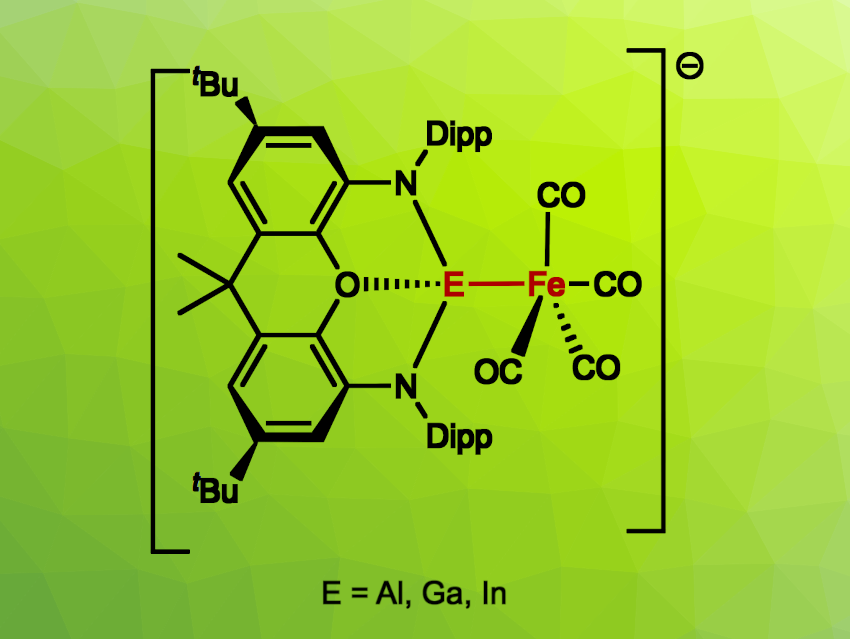
Simon Aldridge, University of Oxford, UK, and colleagues have synthesized a series of such metal trielyl complexes, namely, iron aluminyl, gallyl, and indyl complexes of the type [(NON)EFe(CO)4]– (E = Al, Ga, In; NON = 4,5-bis(2,6-diisopropylanilido)-2,7-di-tert-butyl-9,9-dimethyl-xanthene). The complexes were obtained from Fe(CO)5 via CO ligand substitution and studied using crystallographic and IR/Mössbauer spectroscopic techniques, and their bonding situations were investigated using computational methods.
The team found that for the aluminyl system, the reaction outcome depends on the availability of the counterion, a potassium cation. When the crown ether 18-crown-6 was used to capture the K+ ion, a complex featuring a direct, unsupported Al–Fe bond was obtained. With 2.2.2-cryptand, the researchers obtained a species with carbonyl ligands bridging the Al–Fe bond. In the absence of such agents, the product features no Al–Fe bond, but has a structure in which two [Fe(CO)4] units are linked by a pair of [(NON)Al] fragments to form a twelve-membered macrocyclic structure, with the metal centers separated by CO units. For the heavier elements gallium and indium, Fe–E bonded adducts are formed in a straightforward manner.
- Synthesis, and Structural and Spectroscopic Analysis of Iron‐Trielyl Complexes,
Liam Griffin, Alexis Bauer, Agamemnon Crumpton, Mathias Ellwanger, Andreas Heilmann, Anja Wiesner, Michael Neidig, Simon Aldridge,
Chem. Eur. J. 2025.
https://doi.org/10.1002/chem.202404451