Metal-catalyzed carbon–carbon bond activations are useful tools in organic synthesis. For cycloaddition reactions, low-valence metal catalysts can be used. The use of high-valent metal catalysts such as Rh(III) species to trigger cycloaddition reactions via a C–C bond activation has remained challenging so far.
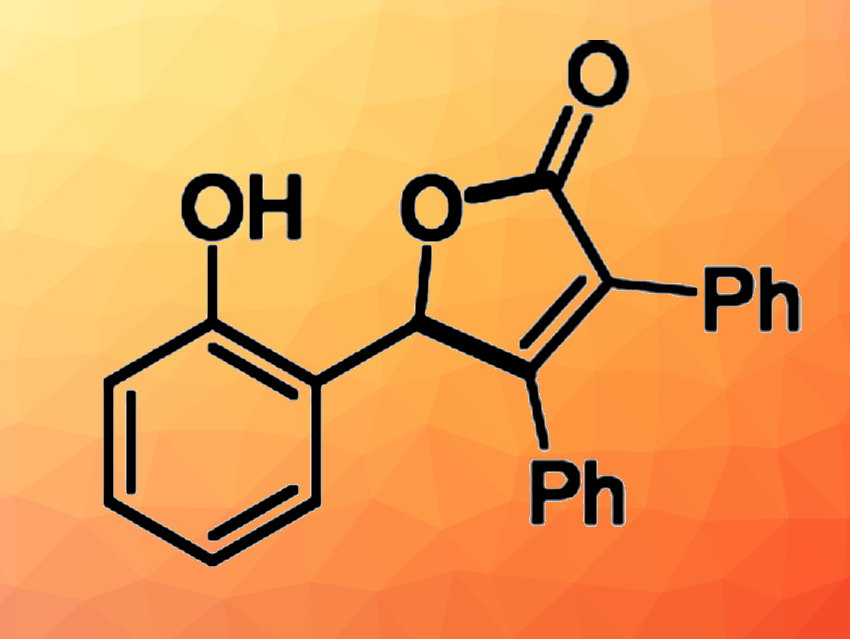
Meng Sun, Northwest University, Xi’an, China, and colleagues have developed a Rh(III)-catalyzed formal cycloaddition reaction between cyclopropenones and aldehydes that gives highly functionalized furanones (example product structure pictured). The team used [Cp*RhCl2]2 as the catalyst (CP* = 1,2,3,4,5-pentamethylcyclopentadiene) and NaOAc as a base to react a range of ortho-hydroxybenzaldehydes with different cyclopropenones at 50 °C.
Under these conditions, the desired furanones were obtained in high to excellent yields. The researchers propose a reaction mechanism that involves a C–H bond activation at the aldehyde to form a five-membered rhodacycle intermediate. The cyclopropenone coordinates to this species and a tripodal Rh–carbene intermediate is formed. A migration insertion step and a protonation then lead to the product. Overall, the developed reaction offers a broad substrate scope, good functional group compatibility, and high atom economy.
- Rh-Catalyzed Formal [3 + 2] Cycloaddition Reactions with Cyclopropenones via Sequential C–H/C–C Bond Activation,
Qing-Long Qu, Yu-Tong Ren, Jun-Tao Cao, Wei Sun, Meng Sun,
Org. Lett. 2025.
https://doi.org/10.1021/acs.orglett.5c00248