3D printing technology is expanding the possibilities for designing and manufacturing plastic components. However, a shortage of high-performance recyclable polymers remains a challenge. Researchers have developed a new class of polythioenones (PCTE) synthesized via Michael addition-elimination ring-opening polymerization (MAEROP) of cyclic thioenone (CTE) monomers, which are both mechanically and chemically recyclable and suitable for 3D printing. The polymers also demonstrate better mechanical properties than conventional polyolefins—thanks to the special, ring-shaped building block.
3D Printing
Using digital modeling, complex structures can be precisely constructed layer by layer with 3D printers. Extensive customization and rapid prototyping open new possibilities in fields such as biomedical engineering, automotive manufacturing, and consumer product design. In 3D printing with the fused filament fabrication (FFF) process, a threadlike thermoplastic material is pressed through a hot nozzle, where it melts. It is then applied in layers until the desired three-dimensional component is produced—with a minimal waste of material.
The downside to this method is the lack of suitable polymers that can be recycled. Chemically recyclable polymers, which can be split apart into their building blocks and repolymerized, would help reduce environmental problems resulting from long-lasting plastic waste and help conserve fossil-derived feedstocks.
Thermoplastic Polymers with Improved Recyclability
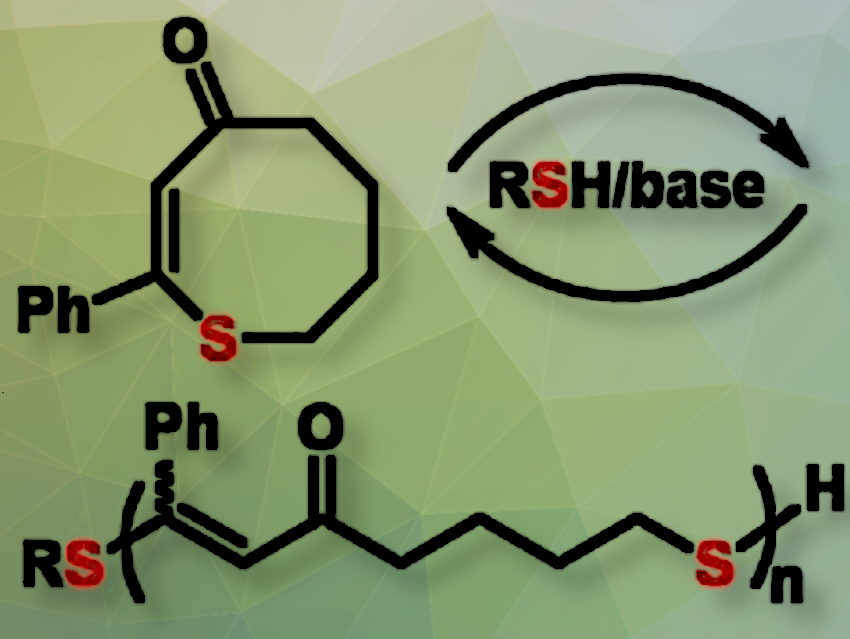
The synthesis of novel thermoplastic polymers with improved recyclability begins and ends with the design of suitable monomers. A team led by Will R. Gutekunst and H. Jerry Qi at the Georgia Institute of Technology, Atlanta, USA, has developed a novel family of monomers: cyclic thioenones (CTE), rings made of seven carbon atoms and one sulfur atom. The rings contain one C=C double bond and one carbonyl group (C=O) and can readily be modified by the addition of different side groups.
The monomers can be polymerized through a ring-opening reaction in which monomers are added one by one to the end of the growing chain. Known as a thia-Michael addition, this reaction is reversible, meaning that the resulting polythioenones (PCTE) can be depolymerized back to the starting monomer.
PCTE-Ph: A Polythioenone with a Phenyl Ring Side Group for Enhanced Performance
One of the synthesized polymers, PCTE-Ph proved to be particularly interesting. It is made from a CTE-monomer with a phenyl ring (Ph) as its side group. PCTE-Ph is a thermally stable thermoplastic with outstanding mechanical properties. Colorants and fillers can be incorporated, and it can be processed by customary methods.
According to the researchers, this new material is especially well suited for 3D printing. Components printed with this material can be mechanically recycled by simply melting the material and processing it again while maintaining its advantageous properties, such as tensile strength and thermal stability. In addition, it can be catalytically depolymerized back to the initial monomer in 90 % yield. This recovered monomer is then available for additional rounds of polymerization.
- Reprocessable and Recyclable Materials for 3D Printing via Reversible Thia-Michael Reactions,
Yong-Liang Su, Liang Yue, McKinley K. Paul, Joseph Kern, Kaitlyn S. Otte, Rampi Ramprasad, H. Jerry Qi, Will R. Gutekunst,
Angewandte Chemie International Edition 2025.
https://doi.org/10.1002/anie.202423522