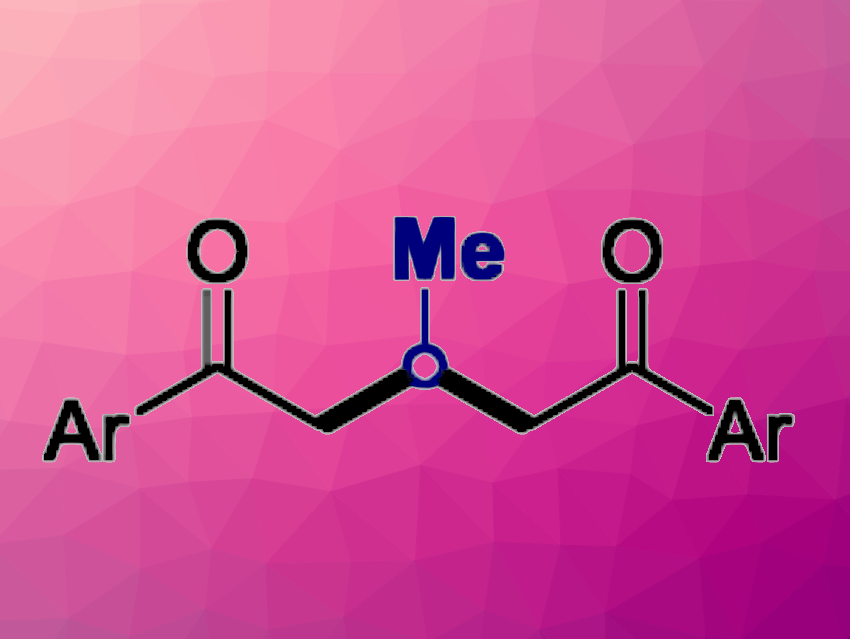
1,5-Diketones are often found in natural products or drugs, and they can serve as valuable intermediates in organic synthesis. Sustainable strategies for their preparation are, thus, interesting research targets. The use of ethanol and methanol as C1 and C2 building blocks via dehydrogenation is interesting in this context.
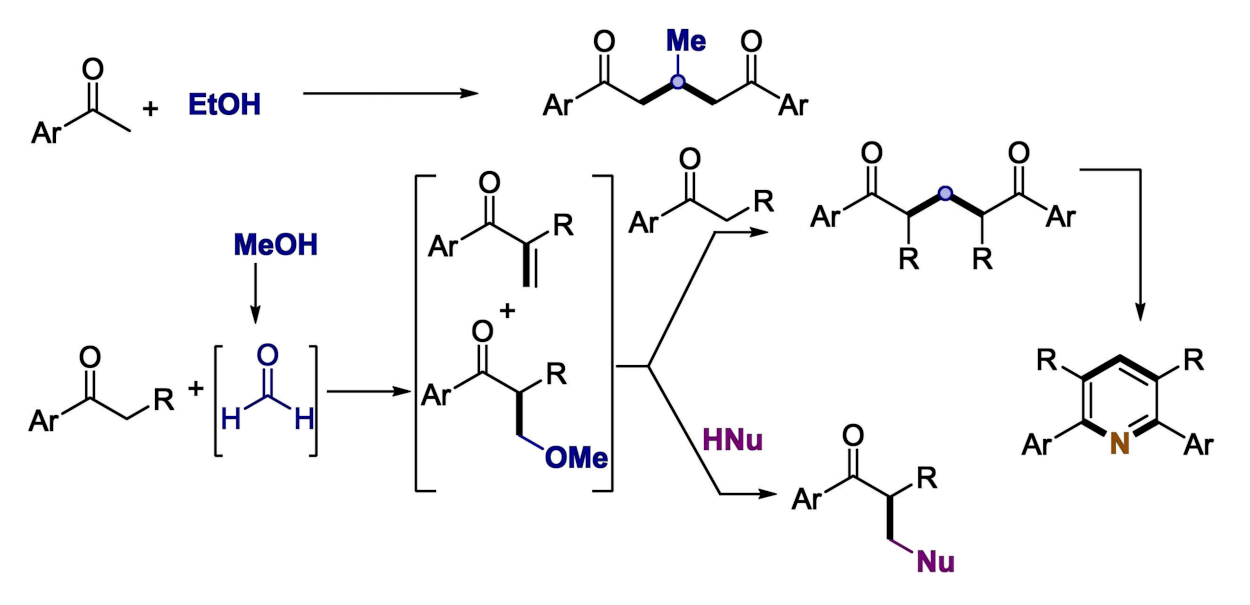
Debasis Banerjee, Indian Institute of Technology Roorkee, and colleagues have developed a nickel-catalyzed strategy for the synthesis of 1,5-diketones from renewable ethanol and methanol via a dehydrogenation and a C−C coupling with ketones. The team used Ni(OAc)2·4H2O or NiCl2·DME (DME = dimethoxyethane) as the catalysts in combination with 1,10-phenanthroline as a ligand, tBuOK as a base, and toluene as the solvent. They obtained the desired 1,5-diketone products in moderate to high yields.
The synthetic strategy can also be used for one-pot α-aminomethylations via the enone using various nitrogen nucleophiles (pictured below in purple). In addition, the protocol can be extended and used, for example, for the one-pot synthesis of tetra-substituted pyridines (pictured below in the bottom right corner). The researchers performed mechanistic studies and deuterium labeling experiments, which showed that the reaction proceeds via an interrupted borrowing hydrogen pathway, i.e., the dehydrogenation takes place, but the hydrogen is not added back to the enone intermediate. The process releases H2 and H2O as by-products.
- C−C Coupling of Ketones with Ethanol and Methanol Enabled by Nickel Catalysis,
Motahar Sk, Adrija Ghosh, Lalit Mohan Kabadwal, Debasis Banerjee,
Eur. J. Org. Chem. 2025.
https://doi.org/10.1002/ejoc.202500067