Thioxanthones have many applications, including in the printing industry, as drugs to treat parasitic infections and cancer, as photocatalysts, or as intermediates in the synthesis of molecular motors. Despite this remarkable versatility, the synthesis of functional thioxanthones can be quite challenging, often requiring multiple complex steps under precise conditions.
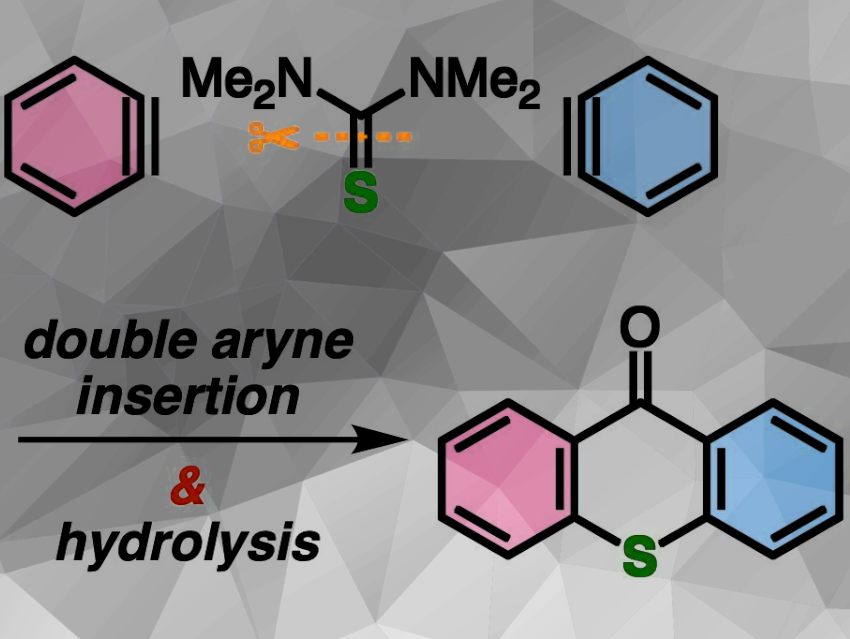
Suguru Yoshida and colleagues, Tokyo University of Science, Japan, have developed a novel method to prepare complex thioxanthones from relatively simple and accessible precursors. The researchers started with o-silylaryl triflates and thioureas. Their strategy involves the double aryne insertion into the C═S double bond of thioureas, followed by hydrolysis to form thioxanthones. Nucleophilic sulfur acted as the S2– equivalent, and electrophilic carbon as the carbonyl source. After testing various reaction conditions, the researchers identified N,N‘-dimethylthiourea as the compound that led to the best yields.
The team successfully synthesized a broad range of thioxanthones, including tetrasubstituted, asymmetric, multisubstituted, and even highly challenging π-extended thioxanthones, with good functional group tolerance. This result demonstrates the efficiency and versatility of the method in accessing complex thioxanthone structures.
Since the strategy is straightforward and involves only a few steps, it has the potential to lower production costs for drugs and industrial chemicals derived from multisubstituted thioxanthones. Moreover, the researchers say that the ability to easily synthesize π-extended thioxanthones opens up new avenues for developing materials with enhanced electronic and optical properties. This could lead to more efficient organic semiconductors for electronic devices and improved light-absorbing materials for solar cells.
“Further studies are ongoing in our research group, including the synthesis of various functionalized thioxanthone derivatives, such as unsymmetric thioxanthones, a detailed theoretical analysis of the proposed strategy, and the applications of thioxanthone-derived functional materials, such as thioxanthene-type molecular motors,” Suguru Yoshida said.
- Thioxanthone Synthesis from Thioureas through Double Aryne Insertion into a Carbon–Sulfur Double Bond,
Mayu Kawada, Shinya Tabata, Shinya Tabata, Yukitaka Hoshi, Suguru Yoshida,
Organic Letters 2025.
https://doi.org/10.1021/acs.orglett.4c04490




![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)