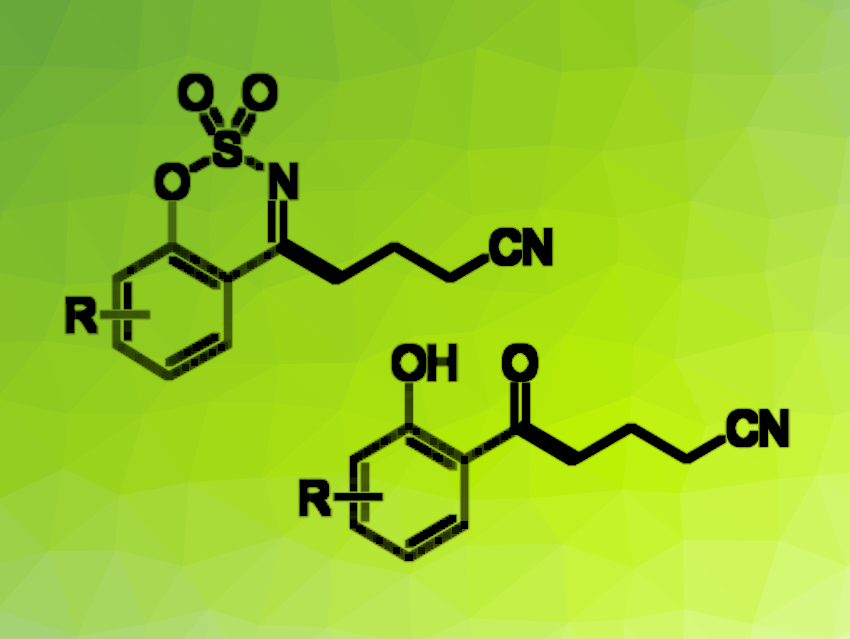
Nitriles are useful intermediates in organic synthesis, as well as often found as structural features in pharmaceutically active compounds. Traditional approaches to the synthesis of nitriles often use toxic cyanide salts, so the development of alternative approaches is helpful.
Teng Wang, Beijing University of Chemical Technology, China, Feng Li, Zaozhuang University, China, and colleagues have developed a method for the silver-catalyzed cross-coupling of cyclic aldimines and α-imino-oxy acids that gives nitrile-functionalized compounds in a solvent-controlled manner (general product structures pictured). When the team used silver nitrate as the catalyst, Na2S2O8 as an oxidant, and an acetone/water mixture as the solvent, they obtained coupling products that retained a cyclic imine unit (pictured above in the top left) in mostly moderate to good yields.
The researchers then changed the reaction conditions, using AgNO3 and Na2S2O8 as before, but replacing the solvent with a dimethyl sulfoxide (DMSO)/water mixture and adding trifluoroacetic acid (TFA). Under these conditions, they obtained ring-opened oxonitriles (pictured in the bottom right), also in generally moderate to good yields. The team proposes a reaction mechanism that involves the generation of an iminyl radical, which is then transformed into a cyanoalkyl radical and attacks the cyclic aldimine. The solvent/additive combination then determines whether an additional hydrolysis step takes place, and thus, which product is formed.
- Solvent-controlled silver catalyzed radical transformation of α-imino-oxy acids with cyclic aldimines,
Jingjing Wang, Yuran Qin, Ke Cui, Xueqi Li, Mingyue Cui, Sheng Cao, Linbo Zhang, Qin Shen, Teng Wang, Feng Li,
Chem. Commun. 2025.
https://doi.org/10.1039/D4CC05675B