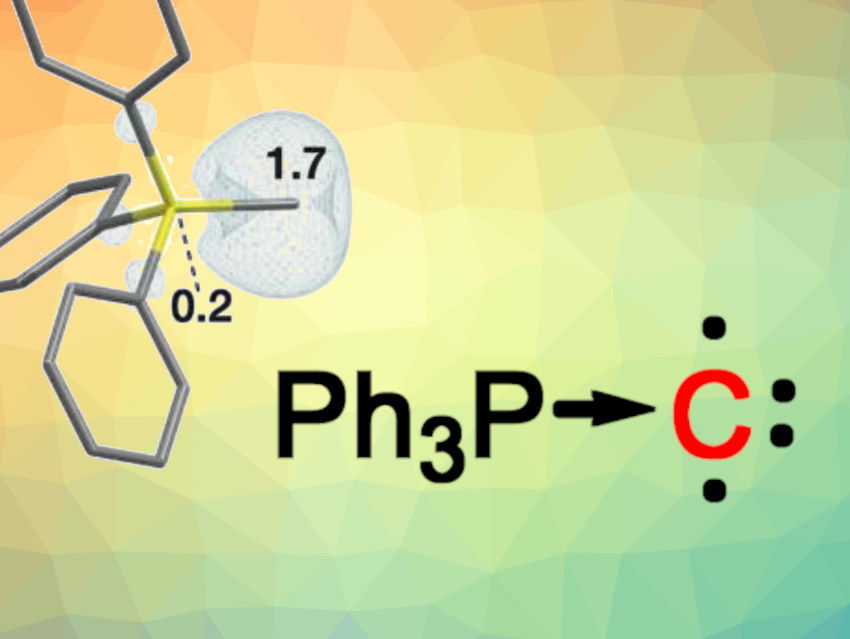
Some compounds that feature non-tetravalent carbon atoms are widely used, such as stable carbenes, while others are part of more rarely observed compound families with unusual chemical bonding situations, such as carbones. Carbones are divalent C(0) compounds in which a central carbon atom is stabilized by two neutral donor ligands, e.g., Ph3P→C←PPh3.
Dimitrios A. Pantazis, Max Planck Institute for Coal Research, Mülheim an der Ruhr, Germany, Müge Kasanmascheff, Max M. Hansmann, TU Dortmund University, Germany, and colleagues wondered what would happen upon a (formal) dissociation of one PPh3 ligand from Ph3P→C←PPh3. Would the resulting Ph3P→C, which features an open-shell monovalent C(0) atom, be stable enough to characterize? Monovalent compounds of other main-group elements, such as triplet nitrenes (R–N) or triplet arsinidenes (R–As) are known, but apart from a triplet vinylidene (R2C=C), compounds with monovalent carbon(0) atoms had remained elusive so far.
The team started from a diazophosphorus ylide precursor, which underwent a UV-light-triggered elimination of N2 under cryogenic conditions. The team used electron paramagnetic resonance (EPR) and electron–nuclear double resonance (ENDOR) spectroscopy to characterize the resulting product, and found that a triplet species had been formed. Quantum chemical calculations were performed to better understand the bonding situation, and the researchers found evidence for the presence of a triplet carbon atom in its ground state that accepts the lone pair from Ph3P and has two unpaired electrons, i.e., the adduct has a diradical character. Thus, the work introduces a new class of carbon-centered diradicals.
- Ph3PC – A Monosubstituted C(0) Atom in Its Triplet State,
Yury Kutin, Taichi Koike, Maria Drosou, Alexander Schnegg, Dimitrios Pantazis, Müge Kasanmascheff, Max M. Hansmann,
Angew. Chem. Int. Ed. 2025.
https://doi.org/10.1002/anie.202424166