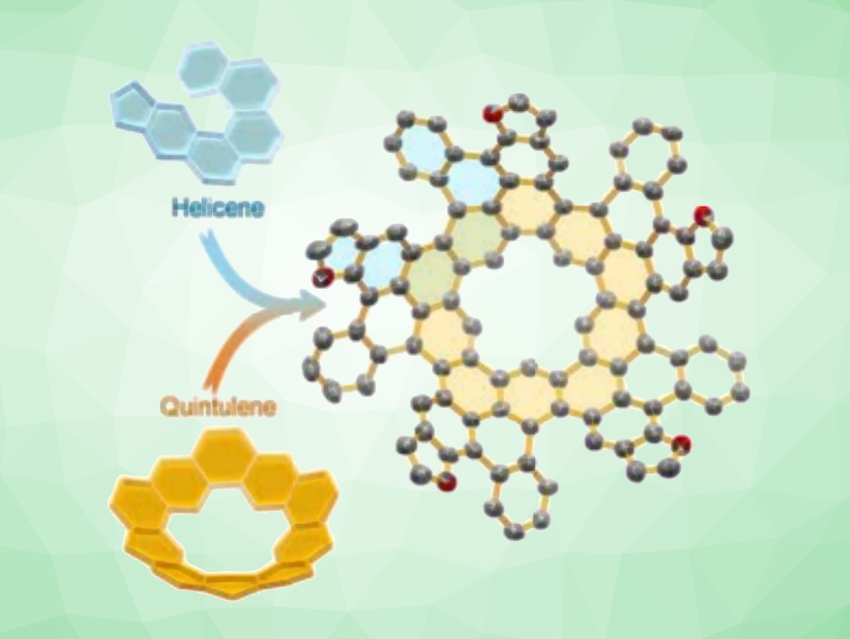
Quintulene is a curved, bowl-shaped cycloarene with a five-fold symmetry. Extended quintulenes have not been well-studied so far. Yuan-Zhi Tan, Xiamen University, China, and colleagues have synthesized an extended quintulene, which features multiple helicene units in its structure (pictured). Helicenes are chiral polycyclic aromatic hydrocarbons (PAHs) with a screw-shaped structure.
The team started by preparing a [5]cyclometaphenylene-based macrocyclic precursor, which was then functionalized via a five-fold Suzuki coupling to obtain an intermediate with five benzofuranyl units. A dehydrocyclization step using 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) was then used to convert this intermediate into the desired product. Benzofuran, which is electron-rich, improves reactivity in the dehydrocyclization and helps to overcome the strain hindering the reaction.
The researchers found that the extended quintulene retains the central bowl-like shape, while the helicene units introduce chirality. The team separated the enantiomers of the product using chiral high-performance liquid chromatography (HPLC). They showed dissymmetric absorption and luminescence properties. The team also found that the bowl-shaped product can form supramolecular assemblies with fullerene. Overall, the work expands the chemistry of quintulenes and multi-helicenes.
- Helical Quintulene: Synthesis, Chirality, and Supramolecular Assembly,
Ling-Xi Huang, Hong-Rui Pan, Jiang-Feng Xing, Xin-Jing Zhao, Ze-Jia Li, Rong-Jie Xie, Yu-Huan Geng, Qing-Song Deng, Yuan-Zhi Tan,
Angew. Chem. Int. Ed. 2025.
https://doi.org/10.1002/anie.202424991

![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)

