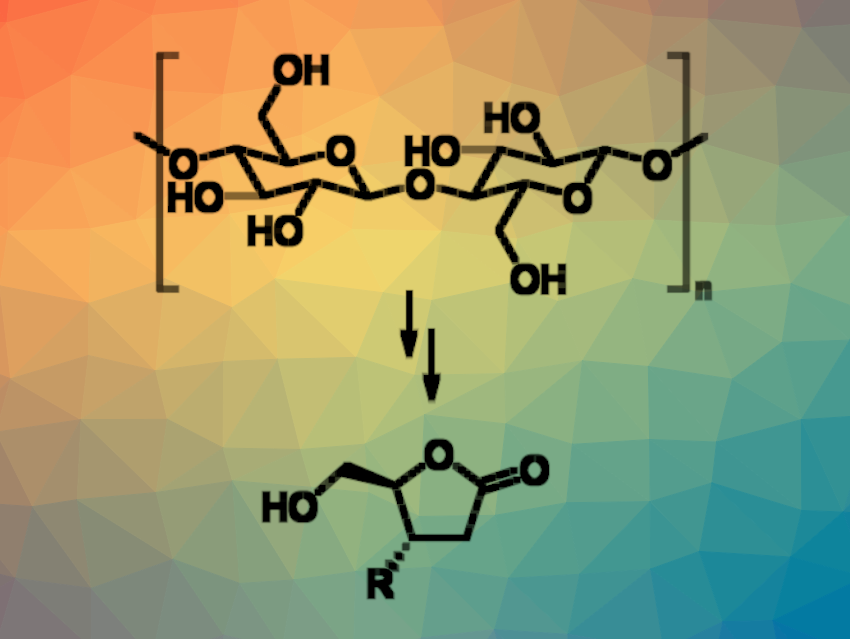
Using biomass for the production of value-added chemicals can be one part of reducing the dependence on fossil resources. Lignocellulosic biomass, for example, is abundant and can serve as a source of sustainable chemicals. It can be used to prepare, e.g., levoglucosenone ((1S,5R)-6,8-dioxabicyclo[3.2.1]oct-2-en-4-one, LGO) via the the acid-catalyzed pyrolysis of cellulose. s. LGO is a chiral bicyclic anhydrosugar containing an α,β-unsaturated ketone with a 1,6-anhydrobridge on one face, which also preserves the “natural” D-stereochemistry of glucose. These features make LGO a potentially versatile chiral precursor for asymmetric synthesis.
Christopher W. J. Murnaghan, Queen’s University Belfast, UK, and colleagues have developed a rapid two-step, one-pot, microwave-assisted method for the Baeyer-Villiger oxidation of biomass-derived levoglucosenone analogues. The team used the acidic resin Amberlyst 15 as a catalyst, hydrogen peroxide as an oxidant, and ethanol as a solvent.
Under these conditions, chiral 5-hydoxymethyl-γ-butyrolactones (general product structure pictured) were obtained in good yields. The reaction is fast, which can be useful to generate compound libraries, and the catalyst can be reused. The chiral butyrolactones can serve as intermediates for further transformations.
- A Two‐step, One‐pot Baeyer‐Villiger oxidation of Biomass‐derived Levoglucosenone Derivatives to Chiral, C4‐Sustituted 5‐Hydroxymethyl Butyrolactones,
Christopher William James Murnaghan, Andrew Cummings, Clare Rice, Gary N. Sheldrake,
Eur. J. Org. Chem. 2025.
https://doi.org/10.1002/ejoc.202401320