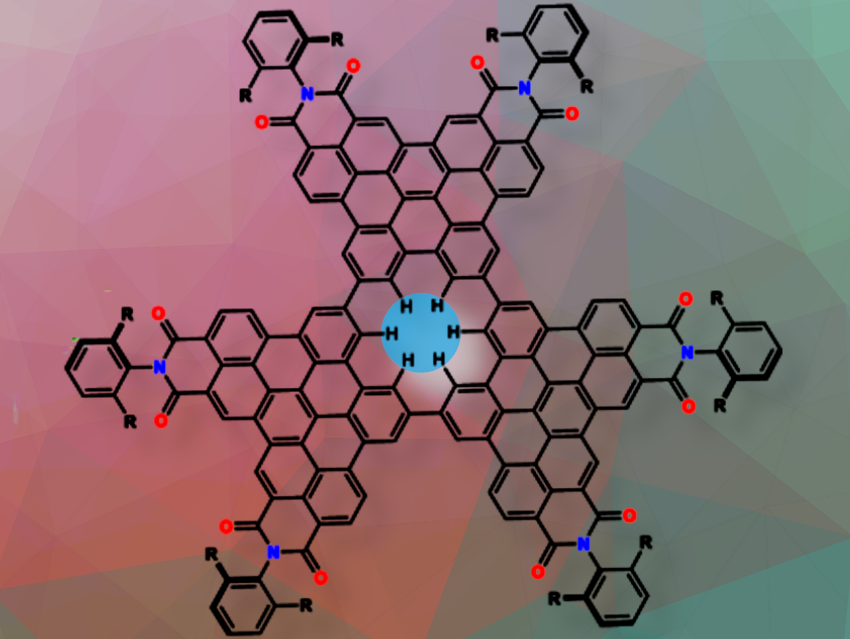
Graphene, a single layer of sp2-hybridized carbon, is impermeable to all atomic entities except hydrogen. The introduction of defects allows selective gas permeation, and the selectivity can be controlled by precise control of the defect size. The permeation of halide ions has potential applications in desalination, detection, and purification, but direct experimental evidence for this process has been lacking. Kazutaka Shoyama, Frank Würthner, and colleagues, Universität Würzburg, Germany, have demonstrated halide permeation through an angstrom-sized single benzene hole in a nanographene (pictured above).
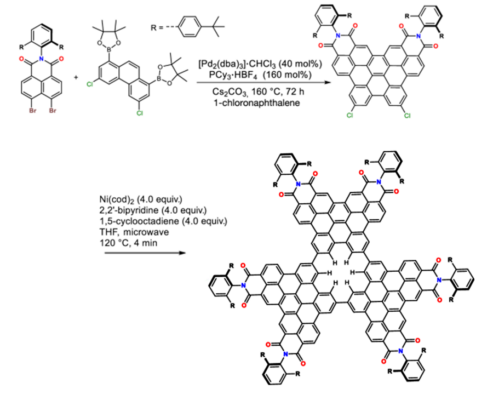
The team synthesized the nanographene using a palladium-catalyzed cascade annulation reaction to form a bisimide precursor, followed by Yamamoto coupling under microwave conditions (see scheme below). Cyclotrimerization of the π-extended phenanthrene units resulted in a single benzene hole in the π-conjugated nanographene structure. To ensure kinetic stability and enable dimer formation, a bulky di-tert-butyl-meta-terphenyl group was attached to the imide nitrogen.
The team found that in solvents such as chloroform, dichloromethane and toluene, the nanographenes are present as monomers. However, in solvents with a higher cohesive energy density, such as dimethylsulfoxide (DMSO), dimethylformamide (DMF) or acetonitrile (MeCN), the nanographene forms stable dimers via strong π-π stacking. The overlapping benzene-sized holes in the dimers form a subnanometre channel surrounded by 12 polarised C-H hydrogens. The atomically precise single benzene pore has a diameter of 1.4 Å.
Halides were trapped in this cavity. Binding affinity tests showed micromolar affinity for fluoride and chloride, millimolar affinity for bromide and impermeability to iodide.
According to the researchers, studying chemical permeability across atomically precise angstrom-sized defects in macroscopic graphene layers using microscopy techniques has been extremely difficult. However, they were able to demonstrate the permeation of halides using the chemical synthesis of a structurally defined nanographene and its supramolecular dimer complex. They anticipate that this supramolecular strategy will be widely used to study the permeation of ionic or molecular entities through atomically precise defects in graphene substructures or, ultimately, graphene itself.
- Bilayer nanographene reveals halide permeation through a benzene hole,
M. A. Niyas, Kazutaka Shoyama, Matthias Grüne, Frank Würthner,
Nature 2025.
https://doi.org/10.1038/s41586-024-08299-8