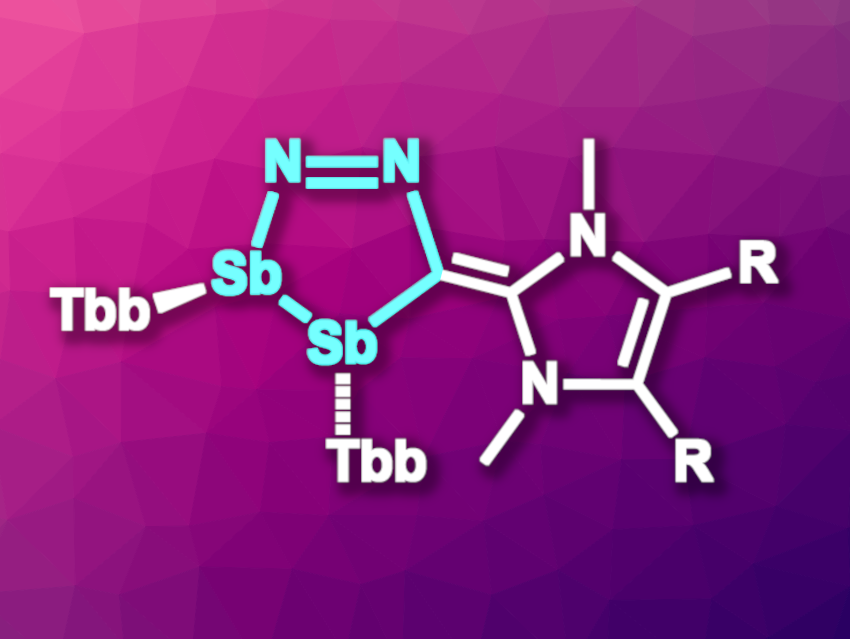
The chemistry of heterocycles with multiple nitrogen atoms is well explored. In contrast, rings that contain heavier elements, such as Sb and Bi, are rarer and can be more challenging to synthesize. The low reactivity of Sb=Sb-type starting materials, for example, hampers the synthesis of antimony-based analogues of 1,2-dihydrotetrazoles.
Antonio Frontera, Universitat de les Illes Balears, Palma, Spain, Alessandro Bismuto, University of Bonn, Germany, and colleagues have synthesized the first diazadistiboylidenes (pictured, R = Me, H; Tbb = 2,6-[CH(SiMe3)2]2-4-(t-Bu)C6H2)), which are heavier analogs of 1,2-dihydrotetrazoles. The team reacted the distibene Sb2Tbb2 with a diazoolefin (R’=C=N2) in a [3+2]-cycloaddition at room temperature. They obtained the desired products in yields of 93 % for R = Me and 80 % for R = H.
The researchers explored the reactivity of the products towards nucleophiles and under visible light irradiation. They found that a reaction with methyllithium gave a rare example of a lithium diantimonide. Under light irradiation, the compounds undergo a transformation to methylenedistibiranes, three-membered rings with one carbon atom and two antimony atoms. These unusual heavy analogues of methylenediaziridines can, in turn, activate CO2.
- Combining Distibene, Diazoolefins, and Visible Light: Synthesis and Reactivity of Inorganic Rings,
Prasenjit Palui, Sangita Ghosh, Rosa M. Gomila, Gregor Schnakenburg, Antonio Frontera, Alessandro Bismuto,
J. Am. Chem. Soc. 2025, 147, 1421–1426.
https://doi.org/10.1021/jacs.4c15626