Nanoconfinement, i.e., processes in which reactions are confined within nanoscale environments, can be used for catalysis. Under nanoconfinement, phenomena such as altered electronic states, enhanced mass and electron transfer, distinct phase behaviors, and modified reaction pathways can occur. This can be useful, e.g., for the degradation of pollutants. However, the controllable synthesis of stable nanoconfined catalysts is still challenging, which limits their scalability and industrial utility.
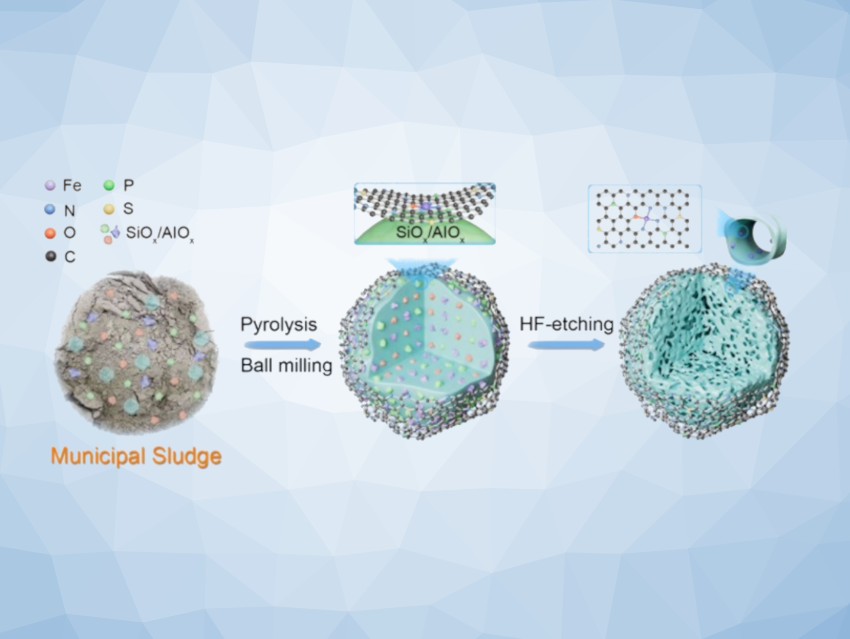
Ai-Yong Zhang, Hefei University of Technology, China, Han-Qing Yu, University of Science and Technology of China, Hefei, Mingyang Xing, East China University of Science and Technology, Shanghai, and colleagues have developed a template-free approach that transforms municipal sludge into high-performance nanoconfined catalysts for ultrafast water purification. Municipal sludge is the semi-solid waste material that is produced as a by-product during wastewater treatment. The nanoconfined catalysts are engineered to optimize reactive oxygen species (ROS) generation.
The team used a three-step strategy to prepare the catalysts, involving pyrolysis, ball milling, and hydrofluoric acid (HF) etching. The sludge was dried and then pyrolyzed at 700 ℃ under nitrogen. The pyrolyzed material was then ball-milled, dried, and etched in a 0.5 M HF solution. The HF etching selectively removes amorphous or low-crystalline SiO2/SiOx/Si and Al2O3/AlOx/Al and creates a structure that confines atomically dispersed Fe centers within a micro-mesoporous carbon matrix.
The developed catalysts show excellent pollutant removal performance, achieving a 261-fold increase in degradation efficiency for compounds such as phenol and sulfamethoxazole compared with unconfined analogs. Overall, the scalable preparation method offers a sustainable route for manufacturing advanced catalysts for water purification.
- Ultrafast Water Purification by Template‐Free Nanoconfined Catalysts Derived from Municipal Sludge,
Chao-Hai Gu, Meng Du, Ru-Yi Han, Ai-Yong Zhang, Han-Qing Yu, Mingyang Xing,
Angew. Chem. Int. Ed. 2025.
https://doi.org/10.1002/anie.202423629