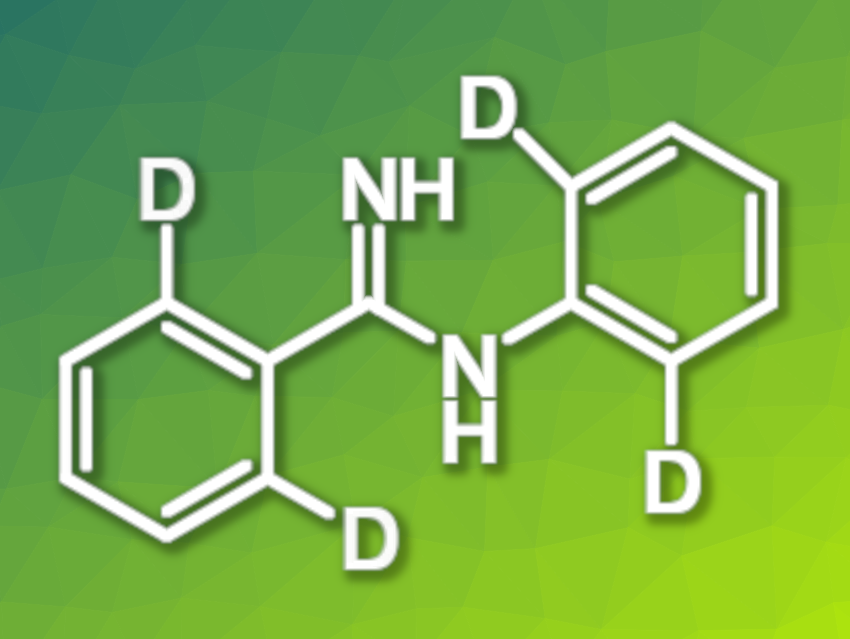
Deuterium-labeled molecules are useful, e.g., in the pharmaceutical industry and materials science due to their difference in properties to the parent compounds. The development of selective deuteration methods for bioactive compoundsis, thus, an interesting research target. Aromatic amidines are often found as a structural motif in pharmaceutically active compounds and can serve as versatile synthetic intermediates.
Jian-Fei Bai, Jia Chen, NingboTech University, China, and colleagues have developed a method for the manganese-catalyzed ortho-selective deuteration of aromatic amidines (simplified product structure pictured), using D2O as the deuterium source. The team used Mn(CO)5Br as the catalyst and NaOAc as an additive, and the deuterations were performed with D2O in N-methyl-2-pyrrolidone (NMP) at 100 °C.
Under these conditions, the ortho-deuteration of a variety of aromatic amidines was achieved with high to excellent yields. The H/D exchange proceeds with high regioselectivity via a five-membered cyclic transition state. The team demonstrated the utility of the method by performing a gram-scale synthesis, obtaining the desired product in a yield of 89 %. The products can be further transformed, e.g., into deuterated triazoles and imidazoles.
- Manganese‐Catalyzed Selective ortho‐Deuteration of Aromatic Amidines with D2O,
Jian-Fei Bai, Hao Hu, Yanran Liu, Zhi-Jiang Jiang, Jia Chen, Zhanghua Gao,
Eur. J. Org. Chem. 2025.
https://doi.org/10.1002/ejoc.202401374