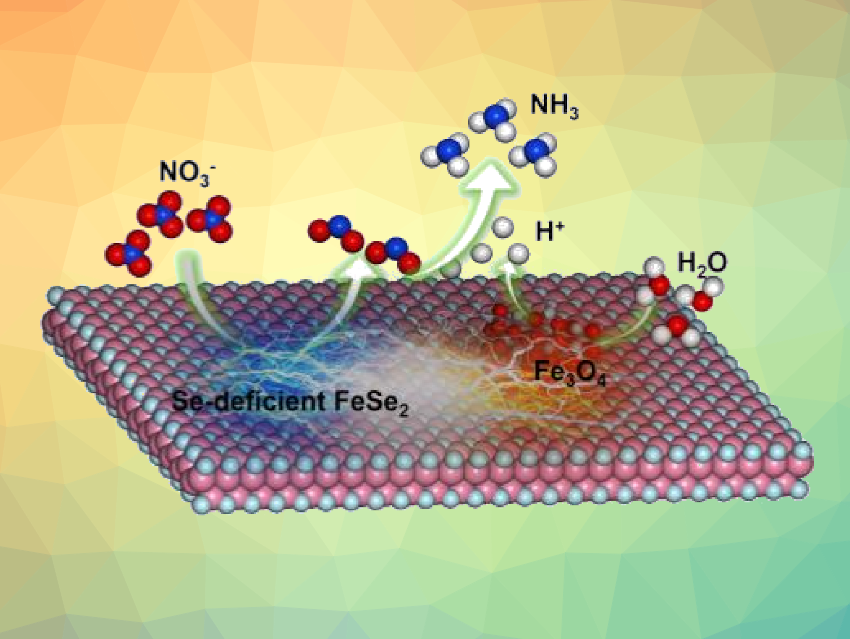
The electrocatalytic reduction of nitrate is a sustainable method to produce valuable chemicals such as ammonia, while simultaneously removing industrial pollutants from wastewater. However, the slow dynamics of the necessary proton-coupled electron transfer process generally result in a high barrier and low conversion efficiency.
Ji-Jun Zou, Tianjin University, China, Yong-Chao Zhang, Qingdao University of Science & Technology, China, and colleagues have developed selenium‐deficient FeSe2/Fe3O4 electrocatalysts for the reduction of nitrate to ammonia. The catalysts were synthesized on pure nickel foam via a one-step hydrothermal method at 180 °C, with different selenium contents giving FeSe1.6/Fe3O4, FeSe1.8/Fe3O4, and FeSe2/Fe3O4-type catalysts. The team found that the electrocatalytic nitrate reduction reaction activity of FeSe1.6/Fe3O4 is higher than those of FeSe2/Fe3O4 and FeSe1.8/Fe3O4.
The researchers attribute this high catalytic activity to synergistic effects between the selenium-deficient iron selenide component and the iron oxide component. The Se-deficient iron selenide contributes to nitrate deoxygenation and the subsequent hydrogenation, while the iron oxide promotes the decomposition of water molecules to give protons. Overall, the developed catalyst has potential for use in the treatment of wastewater with high nitrate concentrations.
- Selenium‐Deficient FeSe2/Fe3O4 Electrocatalyst for Nitrate Reduction to Ammonia,
Yue Du, Haijiao Lu, Jinting Wu, Yalong Zou, Zhen-Feng Huang, Ji-Jun Zou, Tiansheng Mu, Jian Gao, Xiao-Dong Zhu, Yong-Chao Zhang,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202420903