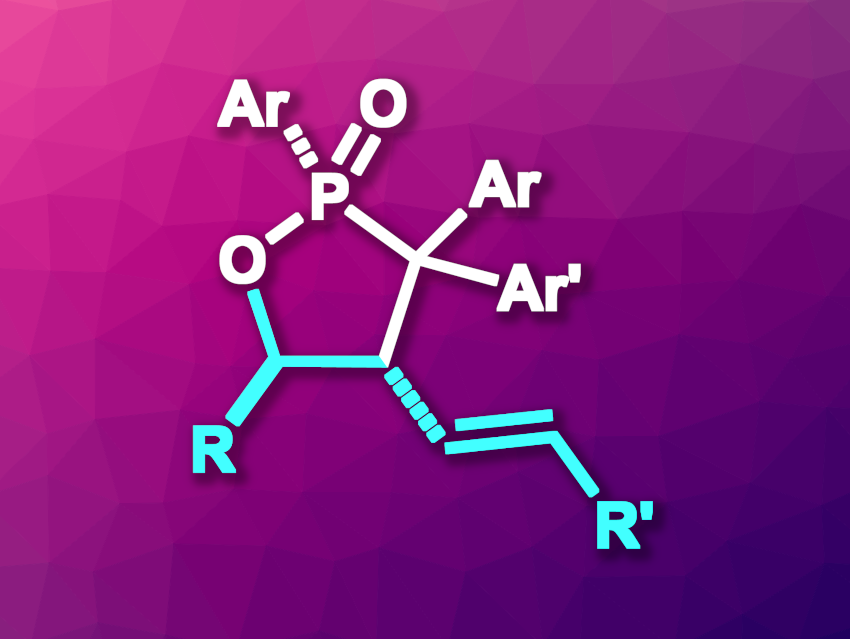
Organophosphorus compounds are useful intermediates in organic synthesis and can also have applications in, e.g., medicinal chemistry, agrochemistry, or materials science. 1,2-Oxaphospholane 2-oxides are five-membered heterocycles with neighboring P and O atoms and can be found as a structural unit in some bioactive compounds.
Jiaxi Xu, Beijing University of Chemical Technology, China, and colleagues have developed a method for the synthesis of vinyl-functionalized 1,2-oxaphospholane 2-oxides (general structure pictured) via a phosphene-induced ring expansion of oxiranes. The team used vinyloxiranes as substrates and phosphoryl diazomethanes as a source of in situ-generated phosphenes. They reacted phosphoryl diazomethanes bearing different aryl substituents with a range of vinyloxiranes in chlorobenzene at 140 °C for 10–20 min.
Under these conditions, the desired 1,2-oxaphospholane 2-oxides were obtained in moderate to excellent yields. The researchers propose a reaction mechanism that involves the formation of a phosphene from the phosphoryl diazomethane, followed by a nucleophilic attack of the oxirane to give a zwitterionic intermediate. Ring opening of the oxirane and another nucleophilic attack then form the five-membered ring. The reaction is metal- and catalyst-free and has a broad functional group tolerance.
- Construction of 4-Vinyl-1,2-oxaphospholane 2-Oxides from Vinyloxiranes and Phosphoryl Diazomethanes,
Yinqiao Wang, Wenlong Tong, Jiaxi Xu,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c04105