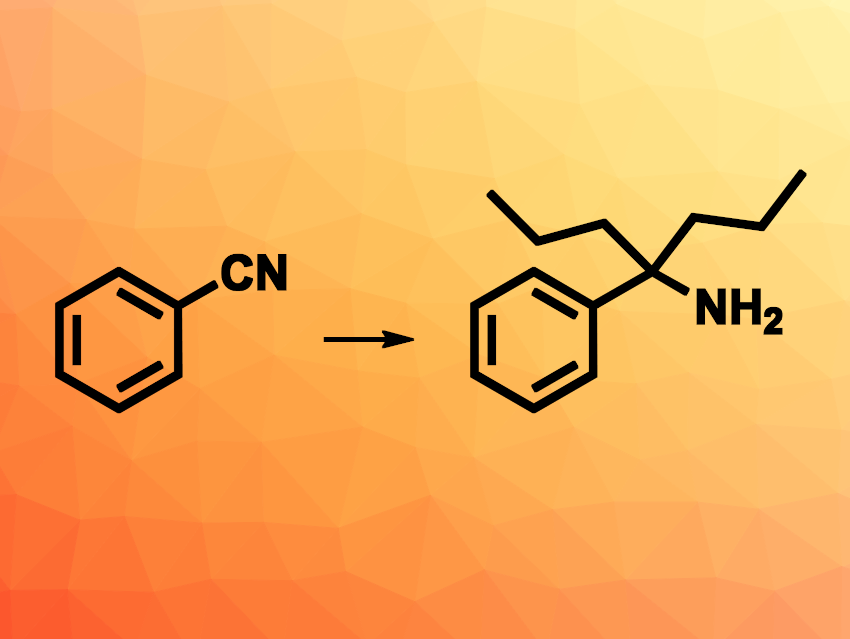
Tert-alkyl amines have a wide range of possible uses, for example, in pharmaceutical chemistry or catalysis. Examples with bulky substituents can be particularly interesting, e.g., as building blocks for ligands. Thus, the development of new approaches for the synthesis of bulky tert-alkyl amines would be useful.
Alexander W. H. Speed, Saint Mary’s University, Halifax, Nova Scotia, Canada, and colleagues have developed a method for the synthesis of tert-alkyl amines with bulky substituents from benzonitriles via the double addition of an ethyl or propyl Grignard reagent (example reaction pictured). The reaction is mediated by a commercially available lanthanum chloride–lithium chloride complex (LaCl3·2 LiCl), which can catalyze otherwise difficult additions of Grignard reagents.
The team first reacted a range of benzonitriles with one equivalent of either EtMgBr or nPrMgCl as the Grignard reagent, then added an LaCl3·2 LiCl solution and two further equivalents of the respective Grignard reagent. The desired products were obtained after hydrolysis in mostly good yields. The substrate scope of the transformation complements other available methods. The products might have applications, e.g., in catalysis.
- Convenient lanthanum-mediated synthesis of bulky tert-alkyl amines from nitriles,
Emily K. Burke, Katherine N. Robertson, Alexander W. H. Speed,
Chem. Commun. 2025.
https://doi.org/10.1039/D4CC05831C