Axially chiral compounds can be useful, e.g., in chiral materials or drug discovery. Synthesizing axially chiral molecules with multiple stereogenic axes could significantly expand the chemical space covered by this type of compound. However, the catalytic stereoselective preparation of axially chiral molecules with more than two axes connected to a single benzene ring has remained challenging so far.
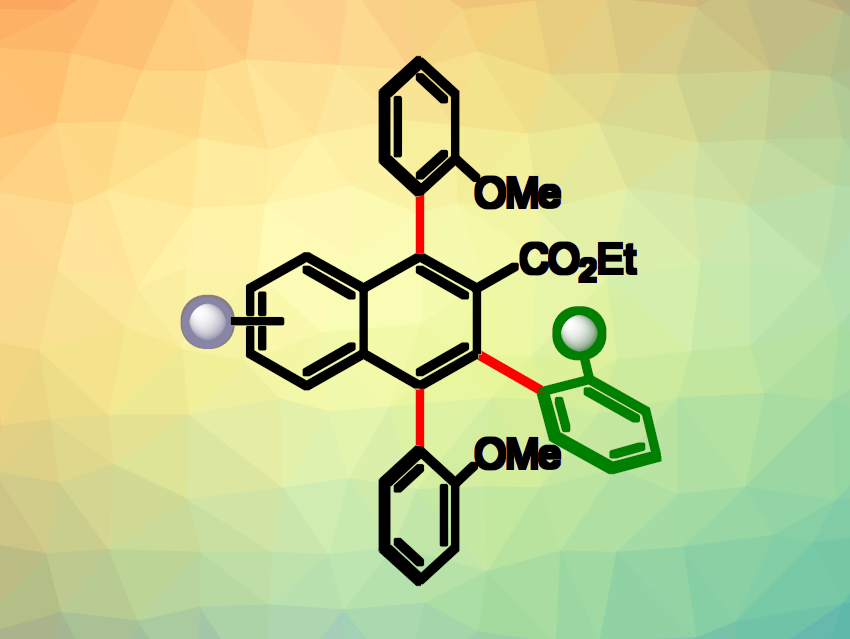
Quan Cai, Fudan University, Shanghai, China, and colleagues have developed a method for the synthesis of triaxially chiral polysubstituted naphthalene derivatives (general structure pictured). The team’s approach is based on a sequence consisting of a Ni(II)-catalyzed Diels–Alder reaction of isobenzofurans and a triflic acid (TfOH)-promoted dehydrative aromatization reaction. They used 1,3-biarylisobenzofuran derivatives and β-aryl-substituted α,β-unsaturated N-acyl pyrazoles as reaction partners in a modular approach.
Via this approach, the researchers obtained a series of axially chiral naphthalene derivatives with three stereogenic axes on one benzene ring (pictured in red) with excellent enantioselectivities and diastereoselectivities. The team successfully performed the reaction on the gram scale, obtaining the desired product in a yield of 76 % and with 92 %[ee]. As an example of potential uses in chiroptical organic materials, they prepared a circularly polarized luminescence (CPL)-active dye with a good luminescence dissymmetry factor and high fluorescence quantum efficiency.
- Enantioselective Synthesis of Atropisomeric Tri‐Axis Naphthalenes via Diels–Alder Reaction and Dehydrative Aromatization of Isobenzofurans,
Yuan-Bo Du, Qi-Tao Lu, Yun-Shu Cui, Kai-Wen Wu, Yu Wang, Yu-Zhen Zhang, Zheng Zhao, Jun-Li Hou, Quan Cai,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202421060