Photoredox catalysis can be useful for the development of new sustainable organic transformations. Hydrocarbons are abundant, but often not well-suited for use as chemical feedstocks due to their low reactivity. Thus, the development of easy-to-use C-H activation protocols under mild conditions for the functionalization of hydrocarbons is an interesting research target. Photocatalysis can help to tackle this problem.
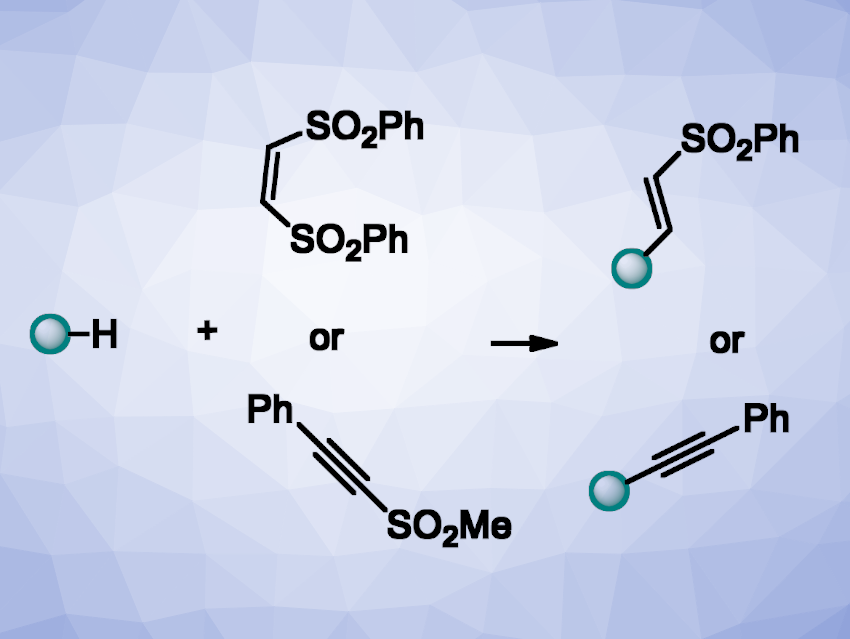
Christoforos G. Kokotos, National and Kapodistrian University of Athens, Greece, and colleagues have developed a light-promoted protocol for the C–H alkenylation and alkynylation of alkanes (general reactions pictured). The team used a CeCl3-based catalytic system together with n-tetrabutylammonium chlorine (TBACl) as a chlorine source to react a range of alkanes with either Z-1,2-bis(phenylsulphonyl)ethylene or [(methylsulfonyl)ethynyl]benzene. The reactions were performed under 390 nm LED irradiation in acetonitrile as the solvent at room temperature.
According to the researchers, the combination of an inexpensive cerium salt with the tetrabutylammonium chloride salt leads to the formation of a cerium(IV) complex, generating a chlorine radical upon irradiation. This chlorine radical reacts with the alkane and initiates a hydrogen atom transfer (HAT) process. The reaction tolerates a variety of linear and non-linear alkanes, as well as bulkier substrates and substrates with different functional groups. The desired alkenylation or alkynylation products were obtained in good to high yields.
- Photocatalytic CeCl3‐Promoted C‐H Alkenylation and Alkynylation of Alkanes,
Naya A. Stini, Petros L. Gkizis, Ierasia Triandafillidi, Christoforos G. Kokotos,
Chem. Eur. J. 2024.
https://doi.org/10.1002/chem.202404063



Nice app