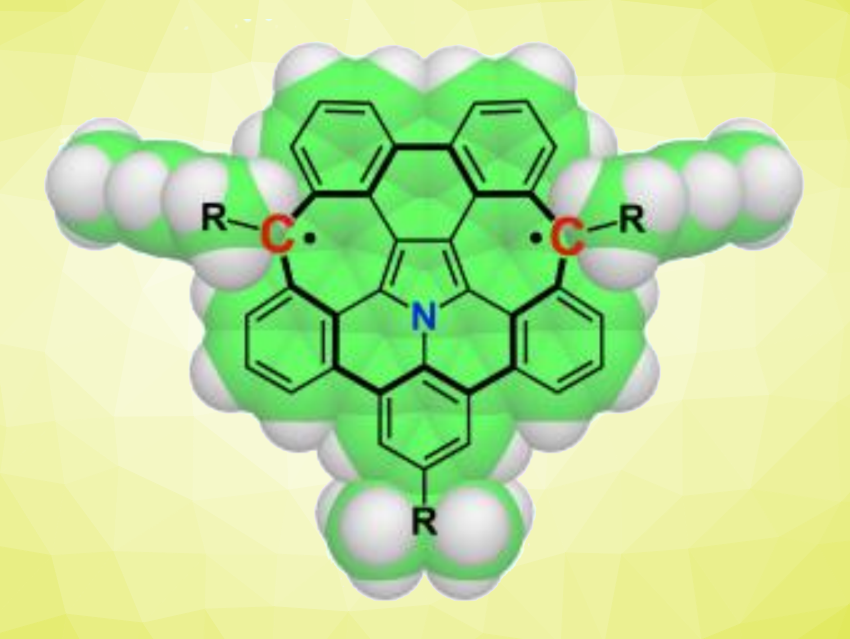
Polycyclic aromatic diradicaloid molecules can have unique electronic and magnetic properties, along with possible applications in functional materials. While diradical(oid) molecules with five-membered rings have been studied extensively, less is known about analogues with seven-membered rings.
Shingo Ito, Nanyang Technological University, Singapore, and colleagues have synthesized two azapentabenzodihomocorannulene derivatives, a dication and a diradicaloid (latter pictured), which contain seven-membered rings. The synthesis uses a mechanochemical C(sp2)–H/C(sp3)–H coupling in the presence of sodium as a key reaction. This coupling leads to intramolecular cyclizations that close the seven-membered rings in a suitable precursor. The resulting intermediate is first transformed to a dicationic species, which can then be reduced to give a neutral product.
Electron spin resonance (EPR) measurements showed that the neutral azapentabenzodihomocorannulene adopts a singlet diradical (diradicaloid) ground state with a small singlet–triplet energy gap of 2.1 kcal/mol. The products were also characterized using X-ray diffraction analysis, which showed that the core of the dicationic form has a twisted structure, while the core of the diradicaloid species is closer to planar, allowing for delocalization of the spin density.
- Azadihomocorannulene as a Heptagon‐Embedded Diradicaloid,
Yosuke Hamamoto, Weifan Wang, Yongxin Li, Shingo Ito,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202416654