Frank Glorius and colleagues, Westfälische Wilhelms-Universität Münster, Germany, have developed a one-pot multi-component alkene-arene and alkene-alkene aminative coupling reaction has been developed for the synthesis of secondary amines and aziridines. In aminative coupling reactions two fragments are assembled directly at the heteroatom.
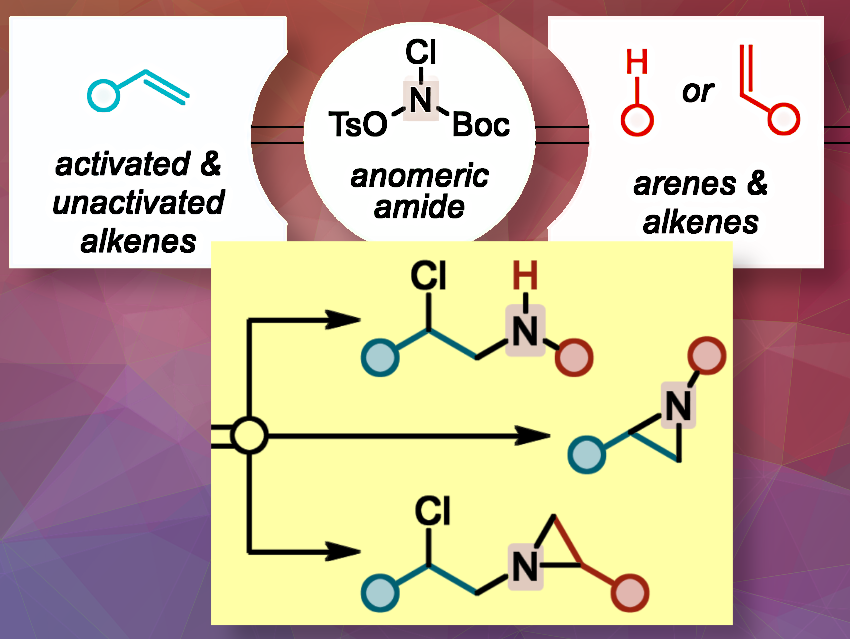
The team has designed and synthesised a precursor, an anomeric amide reagent (tert-butyl chloro(tosyloxy)carbamate), to act as a highly electrophilic N-haloamine. It promotes mild, selective, catalyst-free chloroamination reactions while simultaneously installing a masked nitrene precursor. It could be easily handled and was stored over weeks at −20 °C.
The one-pot aminative coupling combines three steps: First, the anomeric amide reagent reacts with an alkene to form a regioselective 1,2-chloroamine intermediate. Then, the chloroamine undergoes acid-catalyzed deprotection. Finally, this compound reacts further via C(sp²)–H insertion or [2+1] cycloaddition to unify alkene and arene fragments into nitrogen-containing products. A key optimization step is the delayed addition of hexafluoroisopropanol (HFIP). It prevents the decomposition of the anomeric amide before the chloroamine intermediate has formed, allowing the synthesis of the alkene-arene aminative coupling product in 91% yield under mild conditions.
Due to the simplicity of both the protocol and the building blocks required, high-throughput experimentation (HTE) was employed, in combination with a full-scale scope, to rapidly and efficiently explore a wide range of chemical space and determine the limits of reactivity. The method was demonstrated to be robust, tolerant of functional groups, and adaptable to alkene-alkene coupling, providing an efficient route for synthesizing amine-containing products, the researchers say.
- Anomeric Amide-Enabled Alkene-Arene and Alkene-Alkene Aminative Coupling,
Colin Stein, Jasper L. Tyler, Julius Wiener, Florian Boser, Constantin G. Daniliuc, Frank Glorius
Angew, Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202418141