Peter Coburger, Technical University of Munich, Garching, Germany, Hansjörg Grützmacher, ETH Zurich, Switzerland, and colleagues have synthesized a phosphine-stabilized P4C4 cage compound. The creation of this unique cage structure sheds light on an unexplored area of chemistry and provides insights into its reactivity pathways that could be used for future materials design and catalysis.
What have you done?
We aimed to synthesize a specific heterocyclic compound: a planar C₂P₂ four-membered ring stabilized by phosphine substituents on the carbon atoms. This donor-stabilized C₂P₂ ring shows an intriguing electronic structure and can be described as a biradicaloid, with two interacting spin centers located at each phosphorus atom. Fortunately, a suitable precursor for this compound was reported nearly 30 years ago.
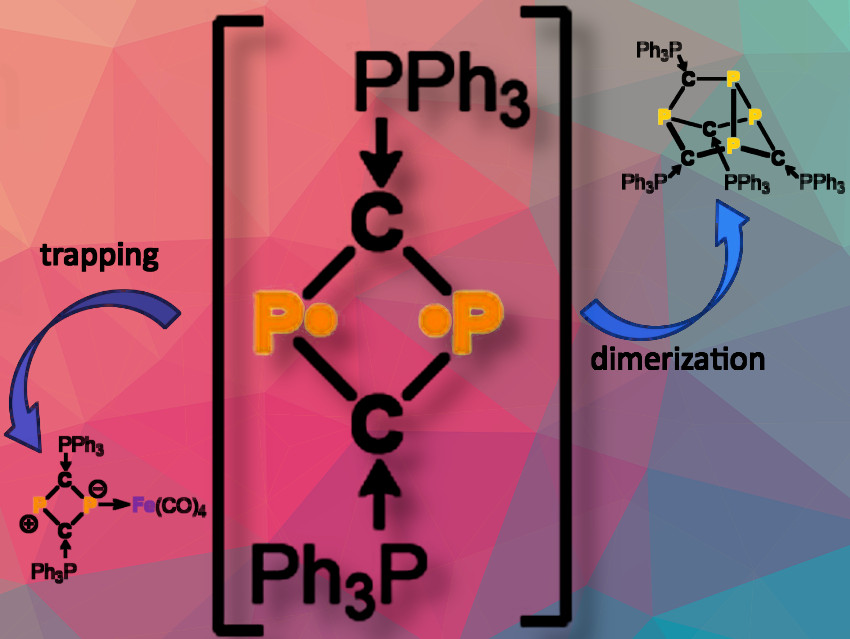
To our surprise, instead of isolating (PPh₃)₂C₂P₂, we observed the formation of a dimer, (PPh₃)₄C₄P₄, which exists in two isomeric forms. One of these isomers is meta-stable and can rearrange into a stable product that we successfully isolated and characterized. We investigated this isomerization using DFT methods.
Interestingly, our research revealed that the target compound (PPh₃)₂C₂P₂ is actually a transient intermediate in the formation of (PPh₃)₄C₄P₄. We confirmed this by trapping the biradicaloid in the form of iron carbonyl complexes.
Why are you interested in this?
This research was motivated by earlier theoretical studies on PₙCₘ phases, which predicted interesting electronic properties for such materials. These properties are eventually linked to carbon nitrides, which are highly attractive materials for energy conversion and storage.
Therefore, we set out to find synthetic access to a molecular C₂P₂ compound which is stabilized by Lewis-bases such as phosphines. Dissociation of the phosphine ligands may lead to PₙCₘ materials, which contain sheets or nanotubes composed from four-membered C₂P₂ rings connected via P-P and C=C bonds.
What is new and especially interresting about this?
We were unable to isolate our target compound, (PPh₃)₂C₂P₂, likely due to inadequate kinetic stabilization from the small PPh3 substituents. However, we successfully demonstrated that this compound is initially formed through our synthetic protocol. This is evidenced by the isolation of a dimerization product and the successful trapping of the C₂P₂ species as an iron carbonyl complex.
These findings confirm that our synthetic approach is a viable method for producing phosphine-stabilized C₂P₂ rings. With appropriately sized phosphine substituents, such compounds may become more accessible in future studies and consequently may indeed serve as precursors for the controlled preparation of PₙCₘ phases.
What is the main significance of your results?
Our research paves the way for two promising lines of research. First, stable, phosphine-stabilized C₂P₂ rings exist as transient intermediates that may become isolable with the introduction of additional kinetic stabilization. Our previous calculations indicate that the phosphine ligands in these C₂P₂ rings are bound much more weakly than, for example, N-heterocyclic carbenes (NHCs), which may facilitate their removal and allow access to “naked” C₂P₂ synthons.
Second, our findings demonstrate that transition metal complexes of small biradicaloids, such as (PPh₃)₂C₂P₂, can be directly synthesized without the need to isolate the biradicaloid itself. This opens up new opportunities for exploring biradicaloid-transition metal complexes and their unique properties.
What specific applications do you imagine?
For the “naked” C₂P₂ rings, we expect the formation of polymeric structures such as sheets and nano-tubes. These are very attractive materials for the development of electrode materials.
For the transition metal complexes, we want to exploit the unique properties of biradicaloids as ligands, such as their strong binding to metal centers, their redox non-innocent behavior, and the presence of Lewis-basic sites. These properties will be used to enhance the catalytic functionalization of olefins with earth-abundant transition metals such as iron and manganese.
What part of your work was the most challenging?
The most challenging part of our experimental investigations was the choice of the correct reducing agent and conditions for (PPh₃)₂C₂(PBr)₂. Many of the initial experiments resulted in inseparable product mixtures. Luckily, after a while, we found the right conditions, which allowed us to finally understand the observed reactivity.
Thank you very much for sharing these insights.
The paper they talked about:
- (Ph3P)4C4P4: Effect of substitution on the Oligomerization of carbon phosphide radicals,
Moritz Scharnhölz, Jose Juan Gamboa Carballo, Nils Trapp, Rene Verel, Peter Coburger, Hansjörg Grützmacher,
ChemistryEurope 2024.
https://doi.org/10.1002/ceur.202400061
Peter Coburger is a Junior Research Group Leader in Inorganic Chemistry with a focus on coordination chemistry and catalysis at the Department of Chemistry at Technische Universität München, Garching, in Germany.
Hansjörg Grützmacher is a research group leader in Inorganic Chemistry with a focus on main group chemistry and coordination chemistry at the ETH Zurich in Switzerland.