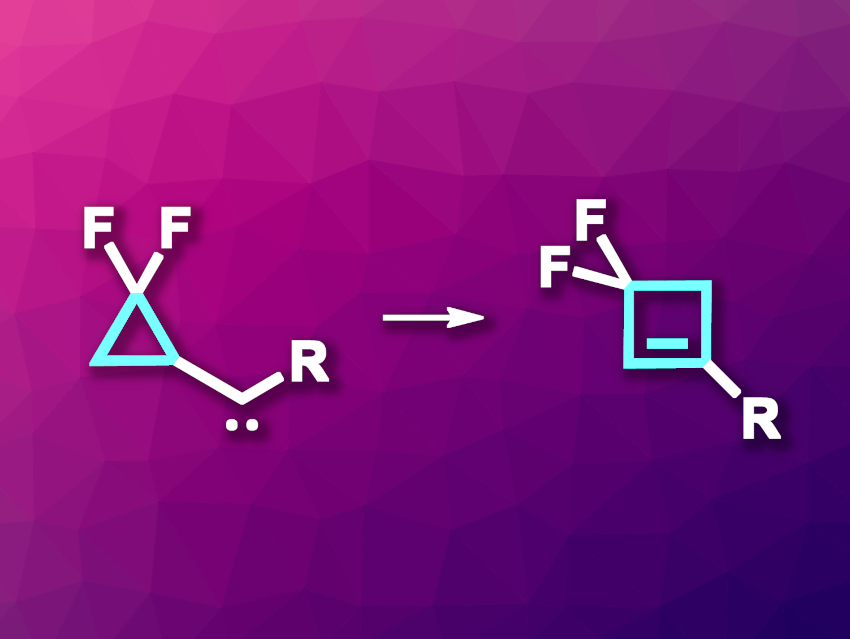
Four-membered rings are found in a number of organic molecules, and they often occur in pharmaceutically active compounds. Introducing fluorine atoms can be useful in drug discovery by modulating, e.g., the stability, lipophilicity, and/or solubility of drug candidates. For example, substructures such as gem-difluorocyclobutyl units exist in some clinically approved drugs. Fluorinated cyclobutenes can serve as useful intermediates, e.g., for the synthesis of such difluorocyclobutane derivatives.
Xufei Yan, Ying Xia, Sichuan University, Chengdu, China, and colleagues have developed a method for the transition-metal-free, fluoro-promoted thermal rearrangement of cyclopropyl carbenes to give gem-difluorinated cyclobutene derivatives (general reaction pictured). The team used gem-difluorinated cyclopropyl N-tosylhydrazones as carbene precursors, which can be synthesized from commercially available gem-difluorocyclopropane carboxylic acid. The cyclopropyl N-tosylhydrazones were converted to the desired carbene intermediates in the absence of transition-metal catalysts using Cs2CO3 as a base and 1,4-dioxane as the solvent. The reactions were performed at 90 °C.
Under these conditions, the desired gem-difluorinated cyclobutene derivatives were obtained in moderate to excellent yields (up to 94 %). The researchers used a variety of different substituents and found that the transformation showed good functional group tolerance. Overall, the work provides a practical path to gem-difluorinated cyclobutenes.
- Fluoro-Promoted Thermal Ring Expansion of Cyclopropyl Carbenes to gem-Difluorinated Cyclobutenes,
Xingyu Qi, Fushan Yuan, Xufei Yan, Ying Xia,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c03920