Ammonia is a very important compound in the chemical industry. Its use in fertilizer production is pivotal for food production. It can also serve as a source of nitrogen atoms for organic synthesis, but this requires methods for the activation of its N–H bonds. This bond activation can be difficult to achieve with transition metals, which often form stable complexes with ammonia.
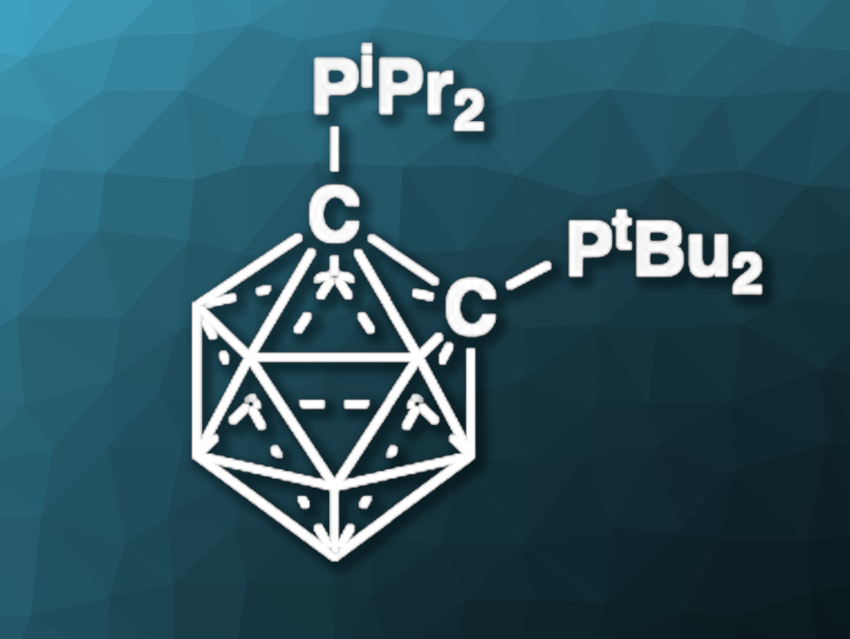
Anthony R. Chianese, Colgate University, Hamilton, NY, USA, Dmitry V. Peryshkov, University of South Carolina, Columbia, USA, and colleagues have developed a new method for the N–H bond activation of ammonia, using the carboranyl diphosphine 1-PtBu2-2-PiPr2–closo-C2B10H10 (pictured). The team bubbled gaseous ammonia through a solution of the carboranyl diphosphine in dimethoxyethane at room temperature and found that a colorless product was formed.
The product was characterized using NMR spectroscopy and X-ray crystallography, which confirmed that the ammonia had been split and that the zwitterionic structure 7-P(NH2)tBu2-10-P(H)iPr2–nido-C2B10H10 had been formed. In this product, one hydrogen atom is bound to one phosphine unit, and the remaining amine group to the other.
According to the researchers, the carborane cluster acts as an electron acceptor, enhancing the electrophilicity of a phosphine unit and allowing for the activation of ammonia via an R3P···NH3 interaction. This is followed by deprotonation of the bound ammonia and a proton transfer. Overall, the work demonstrates the potential of boron-based clusters for promoting metal-free transformations.
- N–H Bond Activation of Ammonia by a Redox-Active Carboranyl Diphosphine,
Amanda L. Humphries, Gabrielle A. Tellier, Mark D. Smith, Anthony R. Chianese, Dmitry V. Peryshkov,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c12146

![Unique Features of the Dinuclear Zirconocene Complex [(Cp₂Zr)₂(µ-Me)(µ-C₂Ph)]](https://www.chemistryviews.org/wp-content/uploads/2025/03/202503_Dinuclear-Zirconocene-Complex-125x94.png)