For the “traditional” conversion of substrates in an electrolyzer, the requirements of electrochemistry (e.g., polar solvents) may restrict outcomes of organic synthesis, and the paired anode and cathode reactions must be compatible. This can limit the control over product selectivity in electrosynthesis, and prevents the formation of some product combinations in paired electrolysis.
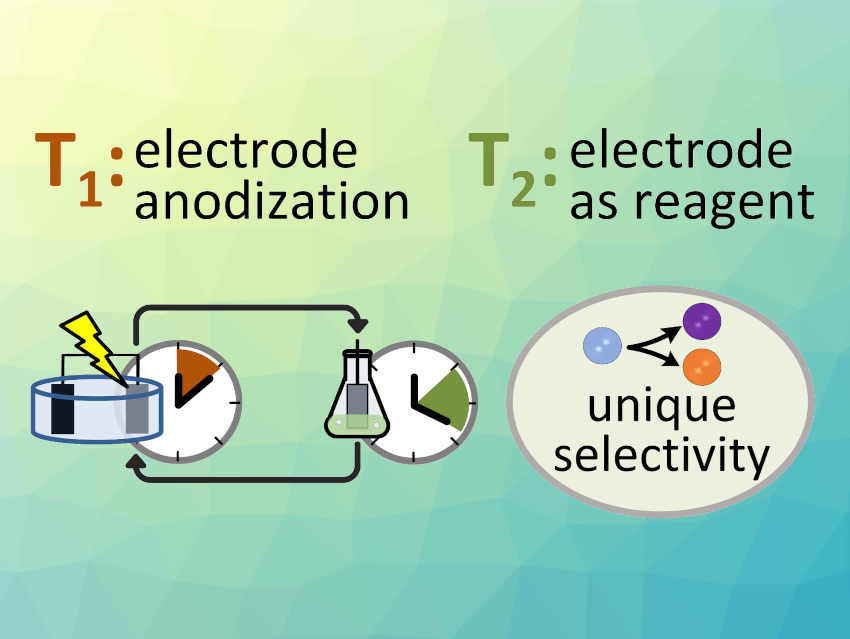
To overcome these problems, Dominik P. Halter, Technical University of Munich, Garching, Germany, and colleagues have developed “ex-situ electrosynthesis”, a new method that decouples the electrochemical catalyst activation from substrate conversion in time and space. The two-step process starts with electrochemical bulk conversion of electrode materials to store redox equivalents. Then, the “charged” electrodes are taken out of the electrolyzer, to drive chemical redox reactions in external vessels under freely tuneable conditions. This enables full control over product selectivity and the free combination of product pairs.
As an example, the team used the method for H2O to H2 reduction paired with benzyl alcohol oxidation. In the presence of water (i.e., in situ), this electro-organic reaction combination can only produce H2 and benzoic acid, whereas using ex-situ electrosynthesis, H2 production with either benzoic acid or benzaldehyde generation is possible. Overall, the new method could allow the synthesis of products that are not easily accessible in situ, unique product combinations in paired electrolysis, and the use of renewable electricity for electrosynthesis at times without wind or sunshine by storing redox equivalents for later use.
- Ex Situ Electro‐Organic Synthesis – A Method for Unrestricted Reaction Control and New Options for Paired Electrolysis,
Helena Pletsch, Yang Lyu, Dominik P. Halter,
ChemElectroChem 2024.
https://doi.org/10.1002/celc.202400489