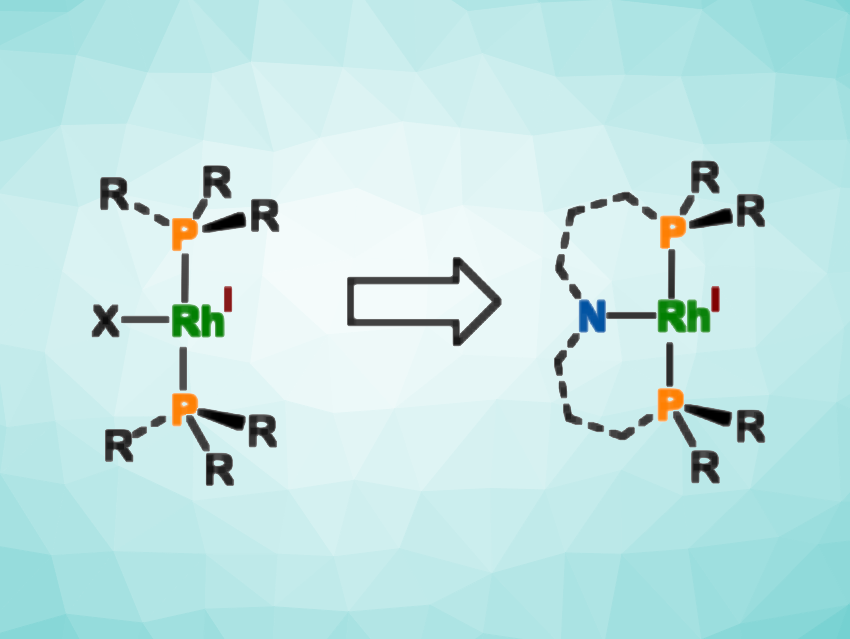
Three-coordinate, T-shaped rhodium complexes with 14 valence electrons are proposed as the active species in some industrial-scale catalytic transformations. Due to their reactive nature, these complexes are generally difficult to isolate and characterize, and known isolable examples lose their catalytic ability, e.g., in the activation of C–H bonds.
Lutz H. Gade, University of Heidelberg, Germany, and colleagues have synthesized two T-shaped 14-electron rhodium complexes that are stabilized using a “framing” PNP pincer ligand (simplified structure pictured above on the right, R = tert-butyl or adamantyl). The team started from the corresponding ligand precursors, which were deprotonated using lithium bis(trimethylsilyl)amide (LiHMDS) and then reacted with a chlorobis(cyclooctene)rhodium dimer ([Rh(COE)2Cl]2) under nitrogen. The resulting dinitrogen complexes were obtained in yields of 96 % for the tert-butyl-substituted ligand and 78 % for the adamantyl-substituted ligand.
In solution, the tert-butyl-functionalized derivative was found to form a cyclometalated Rh(III) hydrido complex that is in equilibrium with the T-shaped 14-electron Rh(I) complex. The adamantyl substituents prevent this cyclometalation and stabilize the T-shaped complex via an agostic interaction with one of the adamantyl C–H bonds. The synthesized complexes can still react with C–H bonds of unactivated substrates such as ethylene and benzene, leading to oxidative C–H addition products.
- T‐shaped 14 Electron Rhodium Complexes: Potential Active Species in C‐H Activation,
Lutz H. Gade, Leon Kambiz Paschai Darian, Joachim Ballmann,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202416814