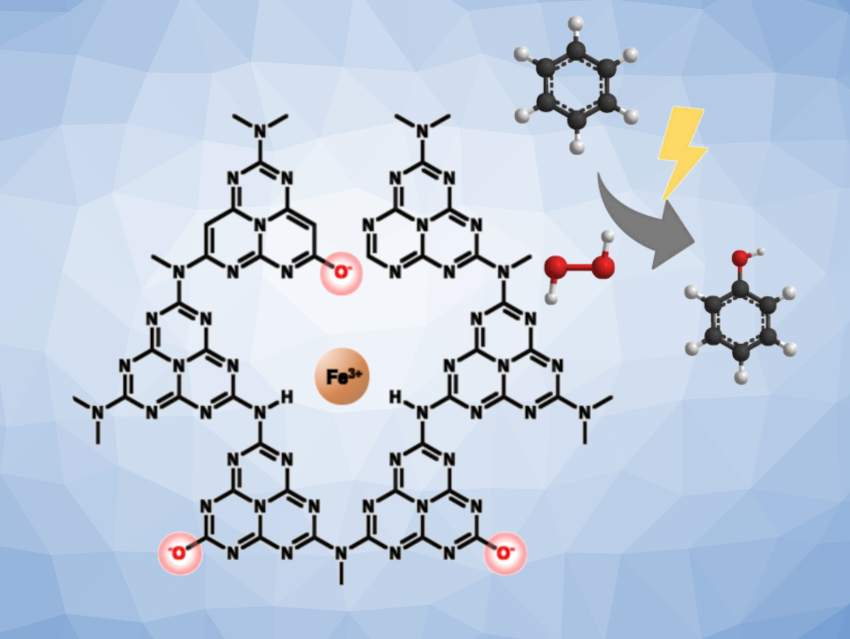
The selective oxidation of hydrocarbons is crucial in the chemical industry, especially for producing high-value compounds such as phenol, which serves as a key intermediate in the production of pharmaceuticals, agrochemicals, and in other sectors. Currently, phenol is synthesized from benzene through an energy-intensive process that involves harsh conditions, hazardous intermediates, and low yields. A more sustainable approach is to achieve phenol production from benzene in a single step via photocatalysis.
Ivo Freitas Teixeira l, Universidade Federal de São Carlos, Brazil, João P. de Mesquita, Universidade Federal dos Vales Jequitinhonha e Mucuri, Diamantina, Brazil, and colleagues have developed a promising material for photocatalytic benzene oxidation. The team used ionic carbon nitrides prepared via the alkaline hydrolysis of polymeric carbon nitrides, which generate charged sites within the structure that stabilize iron species, specifically FeOOH.
The photocatalyst showed exceptional efficiency, achieving a 47 % yield of phenol when using H2O2 as the oxidant, with minimal CO2 by-product formation. Also, the catalyst showed high selectivity towards the hydroxylation of aromatic rings over C(sp3)–H bonds, as demonstrated by the use of alkyl-substituted aromatic substrates.
- Selective Photocatalytic Benzene Oxidation Using Iron‐Carbon Nitride Fragments Functionalized with Cyamelurate‐Like Groups,
Wanessa L. Oliveira, Marcos A. R. da Silva, Gabriel Ali Atta Diab, José Balena, Vitor G. S. Pastana, Luana L. B. Silva, Eduarda Ferreira de Oliveira, Walker Vinícius Ferreira do Carmo Batista, Taís dos Santos da Cruz, Valmor Roberto Mastelaro, Manoel José Mendes Pires, Ivo Freitas Teixeira, João P. de Mesquita,
ChemPhotoChem 2024.
https://doi.org/10.1002/cptc.202400130