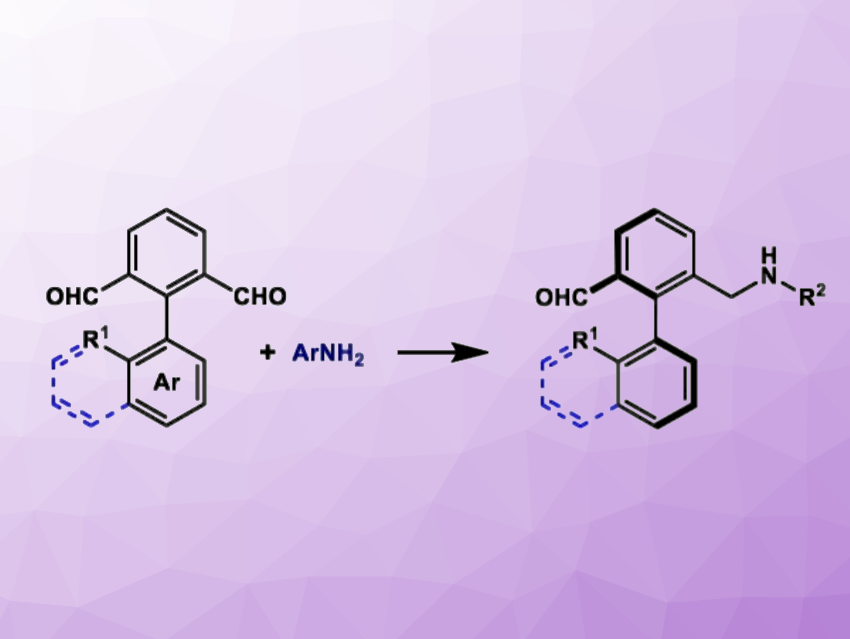
Axially chiral biaryl compounds are a key class of chiral scaffolds with a broad range of applications in the field of asymmetric catalysis, e.g., for drug development. Axially chiral biaryl aldehydes can serve as valuable precursors to new chiral catalysts or ligands. However, effective methods for the synthesis of axially chiral aldehydes are relatively scarce.
Using a strategy based on chiral phosphoric acid catalysis, Xiaofei Zeng, Hangzhou Normal University, Zhejiang, China, and colleagues have developed a method for the synthesis of axially chiral aldehydes from biaryl dialdehydes and aromatic amines (general reaction pictured). The team used an asymmetric reductive amination catalyzed by chiral phosphoric acid, along with tandem desymmetrization and kinetic resolution strategies. They reacted the biaryl dialdehydes with the amines in the presence of the chiral phosphoric acid catalyst using the Hantzsch ester as a hydrogen source and tetrahydrofuran as the solvent. The reactions were performed at room temperature.
Under these conditions, the desired axially chiral biaryl derivatives were obtained in moderate to good yields (up to 82[bnsp]%) and with excellent enantioselectivity (up to 99 % ee). Overall, the work provides a new method for the organocatalytic synthesis of chiral aldehyde derivatives. These products are promising precursors for subsequent chemical transformations.
- Synthesis of Chiral Axially Diaryl Aldehydes by Chiral Phosphoric Acid Catalyzed Desymmetrization Reaction,
Lutong Yuan, Lixin Cui, Yuheng Liu, Wenkai Bao, Qiaohong Zhu, Xiaofei Zeng,
Eur. J. Org. Chem. 2024.
https://doi.org/10.1002/ejoc.202401038



![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)