Dirhodium complexes with a “paddlewheel” structure, such as Rh2(OAc)4, can be used to promote a variety of reactions, e.g., carbene or nitrene transfers, C–H activations, or cycloisomerizations. They can serve as catalysts in redox reactions, both for radical processes and two-electron oxidations. However, two-electron oxidations are less favorable for a dirhodium(II) complex, and fewer examples of this type of reaction are known.
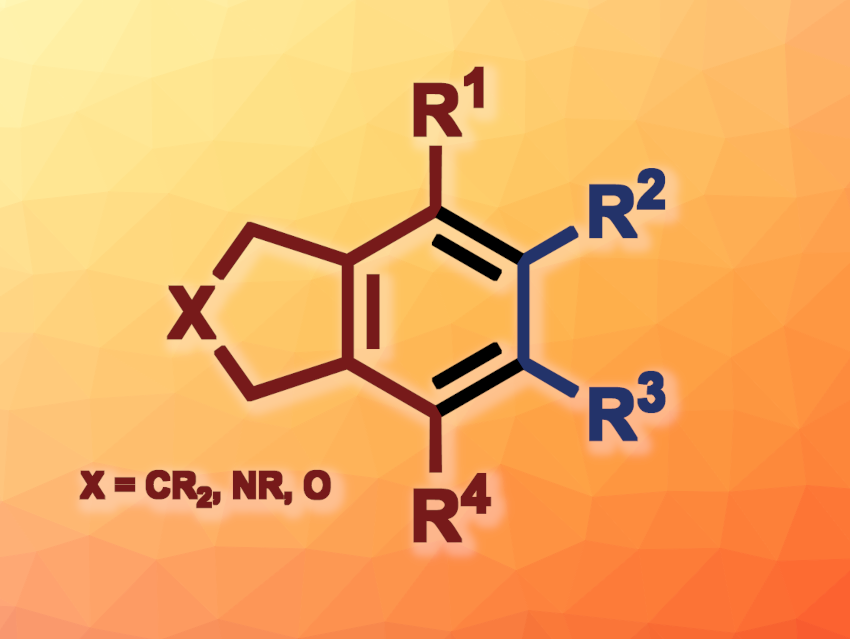
Rui Wu, Zhejiang Sci-Tech University, Hangzhou, China, Shifa Zhu, South China University of Technology, Guangzhou, and Zhejiang Sci-Tech University, and colleagues have developed a method for the ligand-free dirhodium-catalyzed [2+2+2] cycloaddition of 1,6-diynes and alkynes to give fused arenes (general product structure pictured). The team reacted a variety of 1,6-diynes with a group of the type O, NR, or CR2 in the backbone with different alkynes to obtain dihydrobenzofuran, isoindoline, or indane derivatives, respectively. Rh2(OAc)4 was used as the catalyst (without an additional ligand) and 1,2-dichloroethane (DCE) as the solvent, and the reactions were performed at 80 °C.
Under these conditions, the desired fused arenes were obtained in mostly moderate to good yields. Based on mechanistic studies, the team proposes a reaction mechanism that involves an oxidative cyclization between one rhodium of the dirhodium complex and the diyne, followed by coordination and insertion or [4 + 2] cycloaddition and a reductive elimination to give the product. According to the researchers, the work represents a new application of dirhodium complexes.
- Dirhodium-Catalyzed [2 + 2 + 2] Cycloaddition of 1,6-Diynes and Alkynes,
Xiang Gao, Wendeng Li, Yang Chen, Rui Wu, Shifa Zhu,
J. Org. Chem. 2024.
https://doi.org/10.1021/acs.joc.4c01780