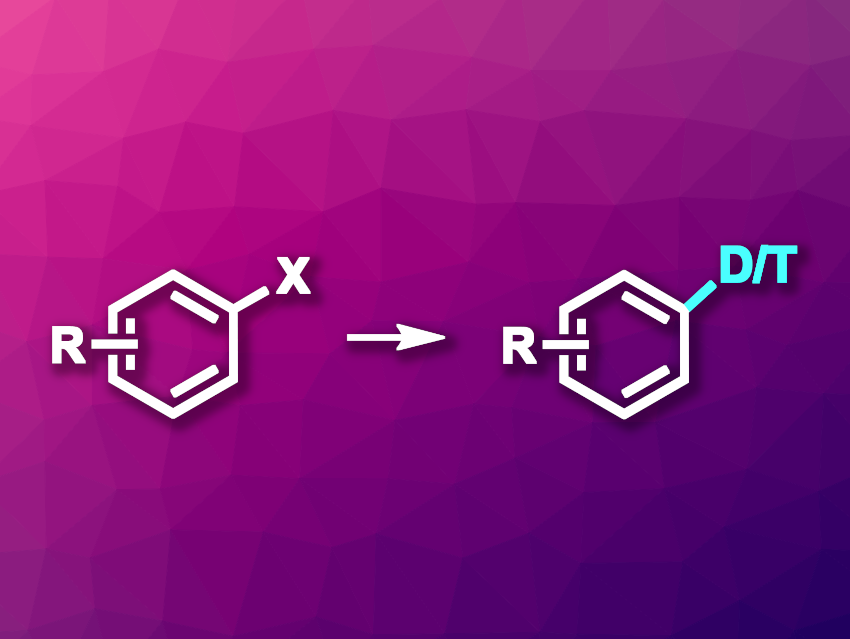
Compounds labeled with heavy hydrogen isotopes, i.e., deuterium or tritium, have important uses, for example, in mechanistic studies or pharmaceutical chemistry. However, it can be challenging to introduce D or T atoms into organic compounds in a regioselective manner and with high conversions. The heterogeneous palladium(0)-catalyzed dehalogenative deuteration or tritiation of aryl bromides and chlorides with D2 or T2 gas, for example, is commonly used, but can have drawbacks such as low functional group tolerance and insufficient yields.
Jingwei Li, Qiao Lin, Merck & Co., Inc., Rahway, NJ, USA, and colleagues have developed a method for the dehalogenative deuteration/tritiation of aryl halides using D2/T2 gas that employs a novel, homogeneous catalytic system and provides high yields and broad functional group tolerance. The team used PdCl2(amphos)2 (amphos = (4-(N,N-dimethylamino)phenyl)di-tert-butyl phosphine) as the palladium catalyst, Zn(OAc)2 as an additive, and dimethylacetamide (DMAc) as the solvent. The reactions were performed at room temperature using D2 or T2 gas.
Under these conditions, a variety of aryl bromides, chlorides, iodides, and pseudohalide-substituted arenes were converted to the desired deuterated or tritiated products in high yields and with mostly quantitative D/T incorporation. The method was successfully used on a gram scale, which demonstrates its potential for use in drug discovery. Overall, the work provides a valuable new method for the deuterium/tritium labeling of arenes or heteroarenes.
- Homogenous Palladium-Catalyzed Dehalogenative Deuteration and Tritiation of Aryl Halides with D2/T2 Gas,
Jingwei Li, Qiao Lin, Otto Dungan, Yue Fu, Sumei Ren, Serge Ruccolo, Sarah Moor, Eric M. Phillips,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.4c08176