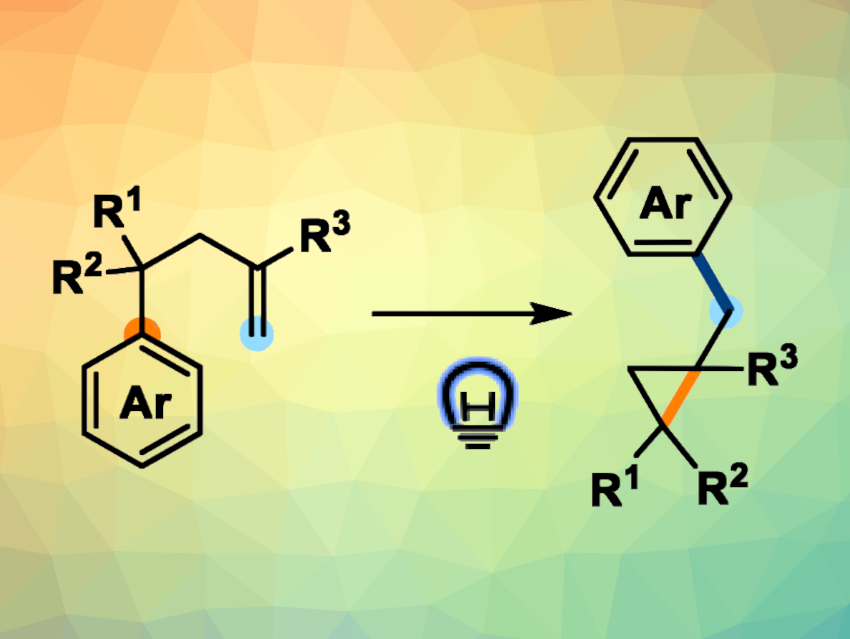
Moving a functional group to a different location in a molecule can be useful in organic synthesis. Aryl group migrations are examples of such functional group translocations. The di-π-methane rearrangement, for example, is a photochemical strategy for moving different functional groups within organic molecules. In this rearrangement, two π-bonds undergo simultaneous migration, leading to the formation of new carbon–carbon bonds and the rearrangement of substituent groups. Visible-light-mediated triplet–triplet energy transfer catalysis (TTEnT) can be used in different photochemical transformations, but its use in rearrangement reactions has been relatively limited so far.
Huan-Ming Huang, ShanghaiTech University, China, and colleagues have developed an unprecedented di-π-ethane rearrangement featuring a 1,4-aryl migration promoted by energy transfer catalysis under visible light (general reaction pictured). The team used an iridium-based photocatalyst, ethyl acetate as the solvent, and 450 nm LED light to convert a range of aryl vinyl ethane substrates into the desired substituted cyclopropanes.
The products were obtained in mostly good to high yields. The method has a broad substrate scope and good functional group tolerance, and due to the mild reaction conditions, it can be used in the late-stage modification of drugs and natural products. The team performed preliminary mechanistic studies, which provided evidence for the involvement of radical species in the di-π-ethane rearrangement and for an energy-transfer mechanism.
- Energy‐Transfer Enabled 1,4‐Aryl Migration,
Shu-Ya Wen, Jun-Jie Chen, Yu Zheng, Jia-Xun Han, Huan-Ming Huang,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202415495


![Synthesis of [c2]Daisy Chains via Mechanochemistry](https://www.chemistryviews.org/wp-content/uploads/2025/04/202504_RotaxanesWithSolidStateMechanochemistry-125x94.png)
