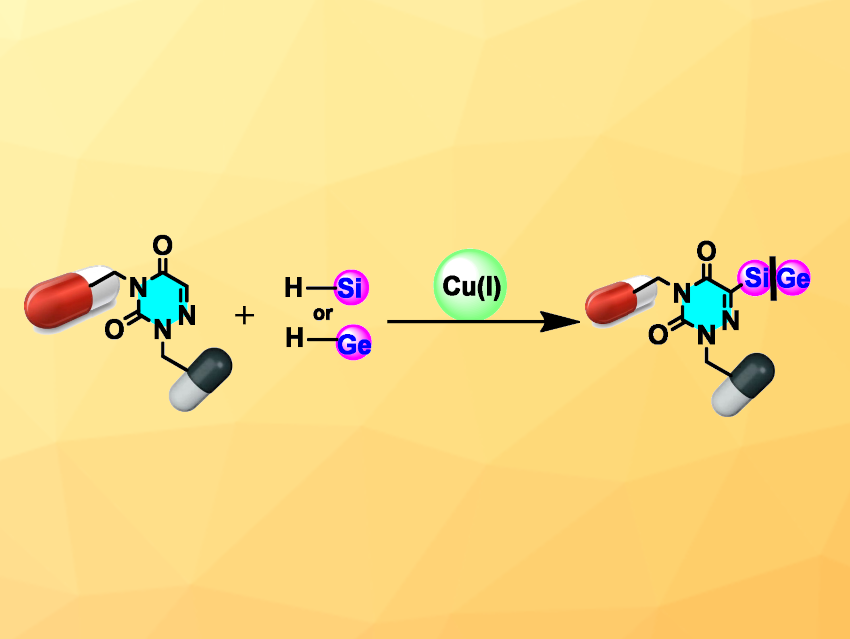
Organosilicon compounds can be useful, e.g., in materials chemistry, polymers, organic synthesis, drug development, and agrochemistry. Analogous, heavier organogermanium compounds can have unique chemical, biological, and physical properties. Still, direct C–H germylation has not been well explored so far. Azauracils are analogues of uracil, one of the four nucleobases in RNA, with an additional nitrogen atom. They are also interesting in the context of pharmaceutical chemistry.
Alakananda Hajra, Visva-Bharati University, Santiniketan, India, and colleagues have developed a method for the Cu(I)-catalyzed direct C(sp2)–H silylation or germylation of azauracils using triphenylsilane or triphenylgermane (pictured). The team used CuBr as the catalyst, tert-butyl hydroperoxide (TBHP) as an oxidant, and acetonitrile as the solvent to react different azauracils with Ph3SiH or Ph3GeH. The reactions were performed under a nitrogen atmosphere at 80 °C.
Under these conditions, the desired silylated or germylated products were obtained in moderate to high yields. The reaction was successfully scaled up to the gram scale, giving a yield of 74 % in an example transformation. The silylated products can also serve as useful precursors for further transformations, such as coupling reactions or deuterations. The team proposes a reaction mechanism involving radical chemistry. Overall, the work provides a path to the divergent functionalization of azauracil derivatives.
- Cu(I)‐Catalyzed Silylation and Germylation of Azauracils,
Frenki Mahato, Asim Kumar Ghosh, Alakananda Hajra,
Chem. Eur. J. 2024.
https://doi.org/10.1002/chem.202403685