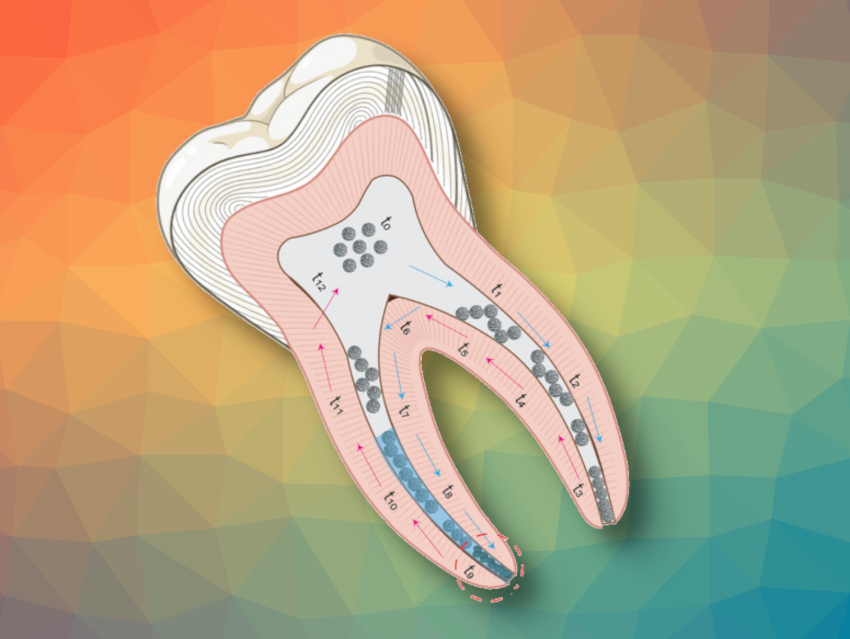
Tooth infections involving microbial biofilms are hard to treat, and root canal systems are challenging to disinfect. Nanozymes, nanoparticles that act like enzymes, can help to break down biofilms. However, current nanoparticles are far from ideal, especially within the confines of root canals, where they may get “stuck” and not reach the most remote sites of infection.
Daeyeon Lee, Edward B. Steager, Hyun Koo, University of Pennsylvania, Philadelphia, USA, and colleagues have developed an improved microparticle with a nanozyme shell containing iron oxide nanoparticles. The team’s new microparticles can be controlled by a rotating magnetic field, and are able to move much more effectively to sites of interest, navigating twists and turns. These “microrobots” can also be removed effectively from the sites of infection.
Current Treatment Options for Biofilms
Microbial biofilms, communities of microorganisms attached to one another and to the surfaces they colonize, cause over 80 % of infectious diseases around the world and are notoriously tricky to break down and treat. One of the most well-known biofilms is dental plaque.
Root canal infections are often caused by biofilms formed in the apical area of tooth roots and are difficult to treat due to the tight twists and turns in these areas. Conventional root canal treatment involves mechanical and chemical disinfection by irrigation with antimicrobial agents such as sodium hypochlorite and ethylenediaminetetraacetic acid (EDTA), but this is usually insufficient to destroy all traces of biofilm and, therefore, fully treat the infection.
Improved Iron Oxide Nanoparticles
As an improvement on the currently-used methods, iron oxide nanoparticles (IONPs) have been proposed. These nanoparticles have strong peroxidase-like activity and break down biofilms by catalyzing the generation of reactive oxygen species (ROS), killing the microbes involved.
Despite their promise, attempts to use IONPs in microrobots have so far been unsuccessful, often leading to aggregation of the particles, which causes blockages that make it impossible to reach most remote sites. In addition, the particles sometimes bind where they should not, leading to further blockages and potentially damaging healthy tissue. If any biofilm is not broken down and remains, infections can recur and are equally difficult to treat.
The team improved on these IONPs and produced their microcapsules by first preparing double oil-in-water emulsions. They mixed a suspension of silica nanoparticles (to stabilize the microcapsules by adsorbing to the phase interfaces), IONPs (for their catalytic biofilm-destroying ability), and toluene (solvent). Evaporation of the toluene left core-shell microcapsules containing the IONPs uniformly distributed throughout the shell.
Making Microcapsules Move
The new microcapsules can be moved to their target location using magnetic fields. When the magnetic moment of the microcapsules aligns with the magnetic field, torque is generated in the microcapsules. The interaction of this torque with fluid drag caused by the substrate on which the microcapsules are located makes the microcapsules roll, setting them in motion.
The team’s microcapsules showed a much-improved ability to navigate the tight spaces and branching structures of root canals. The microcapsules, moving together in a dynamic assembly driven by dipole interactions between adjacent particles, were even able to reconfigure themselves into a zig-zag shape to negotiate curves. Not only that, they could also tackle bifurcated roots, by first rearranging themselves into a suitable shape to enter one branch, then moving back to a central space within the tooth before tackling the second branch.
The team demonstrated that their microcapsules were effective at destroying biofilms. They were able to kill microbial cells in situ by catalyzing ROS generation, and the diffusion of these ROSs into adjacent areas promoted the destruction of the biofilm there, too. The researchers also found that the microcapsules did not significantly interact with any healthy cells, with no binding to other surfaces and no cytotoxicity.
The team envisages using these microcapsules to make root canal infection treatment much quicker and more efficient, targeting the hard-to-reach apical region of roots and preventing recurring infections. They also claim that the coordinated behavior of the microcapsules could help reduce user errors in treating infections by ensuring all areas of the root canal receive treatment.
- Nanozyme‐Shelled Microcapsules for Targeting Biofilm Infections in Confined Spaces,
Hong Huy Tran, Nadasinee Jaruchotiratanasakul, Zhenting Xiang, Nil Kanatha Pandey, Min Jun Oh, Yuan Liu, Zhi Ren, Alaa Babeer, Michael J. Zdilla, David P. Cormode, Bekir Karabucak, Daeyeon Lee, Edward B. Steager, Hyun Koo,
Adv. Healthcare Mater. 2024.
https://doi.org/10.1002/adhm.202402306