Welcome to this week’s research quiz. This time, the focus is on the shortest recorded C(sp3)–I bond, a simple synthesis of 3-substituted bicyclic boronates, and low-cost, real-time multi-metal ion detection.
Challenge yourself with three questions and see how well you’re staying on top of the latest research.
 Question
Question
A better understanding of bond behavior in strained, electron-poor molecules could help chemists design new molecules more effectively. Yuta Uetake, Midori Akiyama, and colleagues in Japan have discovered unusually short carbon–halogen bonds, particularly, the shortest C(sp³)–I bond, in hexafluorodihalocubane compounds.
Which factors contribute to the bond shortening in hexafluorodihalocubanes and the similar halotrinitromethanes?
See answer
In halotrinitromethanes, bond shortening is primarily influenced by the s-orbital character at the carbon atom and hyperconjugation (a type of electron delocalization effect). A higher s-character in a bond orbital increases electron density close to the nucleus, leading to shorter bonds. Hyperconjugation involves electron delocalization from adjacent bonds or lone pairs and can slightly shorten bonds.
In the hexafluorodihalocubanes, Coulombic interactions (i.e., electrostatic forces) between the carbon and the halogen atoms dominate, particularly for the iodine variant, leading to exceptionally short bond lengths. The carbon atom has a partial negative charge, while the halogen atom has a partial positive charge. Iodine’s higher polarizability enhances the electrostatic interaction compared with other halogens.
 Question
Question
Peter H. Seeberger, John J. Molloy, and colleagues in Germany have developed a simple and convergent synthetic path to 3-substituted bicyclic boronates, which are valuable in drug development due to their unique structural and electronic properties.
What makes this new synthetic approach suitable for late-stage functionalizations?
See answer
The approach offers a broad scope and high functional group tolerance, making it adaptable for late-stage functionalizations.
The transformation uses 2-halophenol derivatives and vicinal boronic esters as building blocks and proceeds via a Suzuki–Miyaura cross-coupling/cyclization cascade.
 Question
Question
Lei Wang and colleagues in China have developed a bispyrene-based fluorescent sensor array for the real-time detection of multiple metal ions (Fe³⁺, Cu²⁺, Co²⁺, and Cd²⁺), offering a fast, sensitive, and low-cost alternative to traditional, complex methods. It enables the on-site identification of metal ions through smartphone-based extraction of fluorescence patterns, making it useful for environmental monitoring and public health.
How did the researchers modify the bispyrene derivative to enhance its detection capabilities?
See answer
The researchers functionalized the bispyrene derivative with carboxyl groups, allowing it to bind to different metal ions. This modification altered the compound’s fluorescence signals, creating a unique “fingerprint” for each metal ion.
The News Behind the Quiz
See the news
Cubane Derivatives with Unusually Short Carbon–Halogen Bonds
October 30, 2024
Hexafluorodihalocubanes feature some exceptionally short bonds, including the shortest C(sp3)–I bond found so far
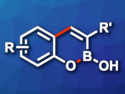 Convergent Path to Bicyclic Boronates
Convergent Path to Bicyclic Boronates
October 25, 2024
Regioselective cross-coupling/cyclization cascade gives useful boron heterocycles
 Fluorescent Sensor Array for the Detection of Multiple Metal Ions
Fluorescent Sensor Array for the Detection of Multiple Metal Ions
October 27, 2024
Bispyrene derivative with aggregation-induced enhanced emission (AIEE) used for the identification of different heavy metal ions

